Prostate Cancer Stem Cells: Research Advances
Abstract
:1. Introduction
2. Stem Cells and Cancer Stem Cells (CSC)
3. The CSC Hypothesis
| Cancer Types | Markers of CSCs | Year of Identification | Reference |
|---|---|---|---|
| Brain tumor | CD133+ | 2004 | [37] |
| Breast cancer | CD44+/CD24− | 2003 | [33] |
| Colon cancer | CD133+ | 2007 | [34] |
| Hepatic carcinoma | CD90+/CD45−/CD44+ | 2008 | [38] |
| Lung cancer | CD133+ | 2005 | [39] |
| Melanoma | ABCB5+ | 2008 | [40] |
| Ovarian cancer | CD44+/CD117+ | 2008 | [41] |
| Pancreatic cancer | CD44+/CD24+/ESA+ | 2007 | [42] |
| Prostate cancer | CD44+/α2β1(hi)/CD133+ | 2005 | [35] |
4. Prostate Cancer Stem Cells
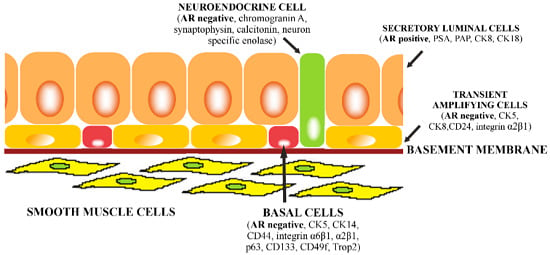
4.1. Prostate Epithelial Stem Cells in the Adult Gland

4.2. Origin of Prostate Cancer
4.3. The Prostate Cancer Stem Cell Niche
4.4. Markers of Prostate Cancer Stem Cells
5. Implications for Prostate Cancer Treatment
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2012, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S.; Bellmunt, J.; Jenkins, C.; Parker, C.; Fitzpatrick, J.M. Perspectives on treatment of metastatic castration-resistant prostate cancer. Oncologist 2013, 18, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Hammerer, P.; Madersbacher, S. Landmarks in hormonal therapy for prostate cancer. BJU Int. 2012, 110 (Suppl. 1), 23–29. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W. Landmarks in non-hormonal pharmacological therapies for castration-resistant prostate cancer. BJU Int. 2012, 110 (Suppl. 1), 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sabbath, K.D.; Ball, E.D.; Larcom, P.; Davis, R.B.; Griffin, J.D. Heterogeneity of clonogenic cells in acute myeloblastic leukemia. J. Clin. Investig. 1985, 75, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Maitland, N.J. Prostate cancer stem cells. Eur. J. Cancer 2006, 42, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Theyer, G.; Steiner, G.; Berg, L.A.; Kozlowski, J.M.; Lee, C. Differential expression of specific cytokeratin polypeptides in the basal and luminal epithelia of the human prostate. Prostate 1991, 18, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; Ramaekers, F.C.; Aalders, T.W.; Schaafsma, H.E.; Debruyne, F.M.; Schalken, J.A. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 1992, 52, 6182–6187. [Google Scholar] [PubMed]
- Bonkhoff, H. Role of the basal cells in premalignant changes of the human prostate: A stem cell concept for the development of prostate cancer. Eur. Urol. 1996, 30, 201–205. [Google Scholar] [PubMed]
- Taylor, R.A.; Toivanen, R.; Risbridger, G.P. Stem cells in prostate cancer: Treating the root of the problem. Endocr. Relat. Cancer 2010, 17, R273–R285. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Ratther, E.; Stylianou, N.; Nelson, C.C.; Hollier, B.G.; Williams, E.D. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: An opportunity for intervention. Front. Oncol. 2014, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, J.; Zhang, R.; Kopecek, J. Combination therapy of prostate cancer with HPMA copolymer conjugates containing PI3K/mTOR inhibitor and docetaxel. Eur. J. Pharm. Biopharm. 2015, 89, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C. Prostate cancer: Radioresistance molecular target-related markers and foreseeable modalities of radiosensitization. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2275–2282. [Google Scholar] [PubMed]
- Tuch, B.E. Stem cells—A clinical update. Aust. Fam. Physician 2006, 35, 719–721. [Google Scholar] [PubMed]
- D’Andrea, V.; Guarino, S.; di Matteo, F.M.; Maugeri Saccà, M.; de Maria, R. Cancer stem cells in surgery. G. Chir. 2014, 35, 257–259. [Google Scholar] [PubMed]
- Fulawka, L.; Donizy, P.; Halon, A. Cancer stem cells—The current status of an old concept: Literature review and clinical approaches. Biol. Res. 2014, 47. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Peng, J.; Zhang, Y.; Cho, W.C.; Jin, K. The implications of cancer stem cells for cancer therapy. Int. J. Mol. Sci. 2012, 13, 16636–16657. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Zhang, Z.; Li, H.; Cheng, W.; Liu, J. Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma. Hum. Pathol. 2013, 44, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; Kaklamani, V.G.; Siziopikou, K. The role of cancer stem cells in breast cancer initiation and progression: Potential cancer stem cell-directed therapies. Oncologist 2012, 17, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xue, F.; Chen, X. Prognostic value of different amounts of cancer stem cells in different molecular subtypes of breast cancer. Gland Surg. 2012, 1, 20–24. [Google Scholar] [PubMed]
- Gudadze, M.; Kankava, Q.; Mariamidze, A.; Burkadze, G. Features of CD44+/CD24-low phenotypic cell distribution in relation to predictive markers and molecular subtypes of invasive ductal carcinoma of the breast. Georgian Med. News 2014, 228, 81–87. [Google Scholar] [PubMed]
- Korski, K.; Malicka-Durczak, A.; Breborowicz, J. Expression of stem cell marker CD44 in prostate cancer biopsies predicts cancer grade in radical prostatectomy specimens. Pol. J. Pathol. 2014, 65, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Fuller, M. Stem cells and cancer: Two faces of eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.A.; Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. The prostate cancer bone marrow niche: More than just ‘fertile soil’. Asian J. Androl. 2012, 14, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Eber, M.R.; Berry, J.E.; Taichman, R.S. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. BoneKEy Rep. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Shaffrey, M.E.; Park, D.M. Cancer stem cells and glioblastoma multiforme: Pathophysiological and clinical aspects. In Advances in Cancer Stem Cells Biology; Scatena, R., Mordente, A., Giardina, B., Eds.; Springer Science & Business Media: New York, NY, USA, 2011; pp. 123–140. [Google Scholar]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.; Leffert, H.L. Liver cancer stem cells. J. Clin. Oncol. 2008, 26, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.; Lam, C.T.; Poon, R.T.; Fan, S.T. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; de Maria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Faja, B.; Cufí, S.; Oliveras-Ferraros, C.; Cuyàs, E.; López-Bonet, E.; Lupu, R.; Alarcón, T.; Vellon, L.; Iglesias, J.M.; Leis, O.; et al. Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle 2013, 12, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar] [PubMed]
- Amorino, G.P.; Parsons, S.J. Neuroendocrine cells in prostate cancer. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 287–300. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Meeker, A.K.; Epstein, J.I.; Coffey, D.S. Prostate stem cell compartments: Expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am. J. Pathol. 1998, 153, 911–919. [Google Scholar] [CrossRef]
- Brawer, M.K.; Peehl, D.M.; Stamey, T.A.; Bostwick, D.G. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res. 1985, 45, 3663–3667. [Google Scholar] [PubMed]
- Alam, T.N.; O’Hare, M.J.; Laczkó, I.; Freeman, A.; Al-Beidh, F.; Masters, J.R.; Hudson, D.L. Differential expression of CD44 during human prostate epithelial cell differentiation. J. Histochem. Cytochem. 2004, 52, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Bello-DeOcampo, D.; Kleinman, H.K.; Deocampo, N.D.; Webber, M.M. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate 2001, 46, 142–153. [Google Scholar] [CrossRef]
- Knox, J.D.; Cress, A.E.; Clark, V.; Manriquez, L.; Affinito, K.S.; Dalkin, B.L.; Nagle, R.B. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am. J. Pathol. 1994, 145, 167–174. [Google Scholar] [PubMed]
- Signoretti, S.; Waltregny, D.; Dilks, J.; Isaac, B.; Lin, D.; Garraway, L.; Yang, A.; Montironi, R.; McKeon, F.; Loda, M. p63 is a prostate basal cell marker and is required for prostate development. Am. J. Pathol. 2000, 157, 1769–1775. [Google Scholar] [CrossRef]
- Wang, Y.; Hayward, S.; Cao, M.; Thayer, K.; Cunha, G. Cell differentiation lineage in the prostate. Differentiation 2001, 68, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.L.; O’Hare, M.; Watt, F.M.; Masters, J.R. Proliferative heterogeneity in the human prostate: Evidence for epithelial stem cells. Lab. Investig. 2000, 80, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Smedts, F.; Debruyne, F.M.; de la Rosette, J.J.; Schalken, J.A. Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate 1998, 34, 292–301. [Google Scholar] [CrossRef]
- Liu, A.Y.; Peehl, D.M. Characterization of cultured human prostatic epithelial cells by cluster designation antigen expression. Cell Tissue Res. 2001, 305, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Hennenlotter, J.; Sobiesiak, M.; Todenhöfer, T.; Scharpf, M.; Stenzl, A.; Bühring, H.J.; Schwentner, C. Surface CD24 distinguishes between low differentiated and transit-amplifying cells in the basal layer of human prostate. Prostate 2013, 73, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Liu, Y.; Liao, L.; Wang, F.; Xu, J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int. J. Biol. Sci. 2014, 10, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.S.; Lawson, D.A.; Cheng, D.; Sun, W.; Garraway, I.P.; Witte, O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Rathore, S.; Goel, H.L.; Li, J.; Alberti, S.; Piantelli, M.; Adams, D.; Jiang, Z.; Languino, L.R. CD133, Trop-2 and α2β1 integrin surface receptors as markers of putative human prostate cancer stem cells. Am. J. Transl. Res. 2010, 2, 135–144. [Google Scholar] [PubMed]
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004, 117, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Kania, G.; Corbeil, D.; Fuchs, J.; Tarasov, K.V.; Blyszczuk, P.; Huttner, W.B.; Boheler, K.R.; Wobus, A.M. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells 2005, 23, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Sykes, A.M.; Huttner, W.B. Prominin-1 (CD133) and the cell biology of neural progenitors and their progeny. Adv. Exp. Med. Biol. 2013, 777, 89–98. [Google Scholar] [PubMed]
- Missol-Kolka, E.; Karbanová, J.; Janich, P.; Haase, M.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Prominin-1 (CD133) is not restricted to stem cells located in the basal compartment of murine and human prostate. Prostate 2011, 71, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Tang, D.G. Cell-of-origin of cancer versus cancer stem cells: Assays and interpretations. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, B.; Beresford, M.; Bowen, R.; Mitchard, J.; Chalmers, A.D. Searching for prostate cancer stem cells: Markers and methods. Stem Cell Rev. 2013, 9, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.M.; Bilen, M.A.; Tannir, N.M. The scientific method: Pillar and pitfall of cancer research. Cancer Med. 2014, 3, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, S.; Loda, M. Prostate stem cells: From development to cancer. Semin. Cancer Biol. 2007, 17, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.M.; Kawasaki, B.T.; Klarmann, G.J.; Thomas, S.B.; Farrar, W.L. CD44+CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer 2008, 98, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Hindoyan, A.; Wang, S.; Tran, L.M.; Goldstein, A.S.; Lawson, D.; Chen, D.; Li, Y.; Guo, C.; Zhang, B.; et al. Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells. PLoS ONE 2012, 7, e42564. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.H.; Frame, F.M.; Collins, A.T. Prostate cancer stem cells. J. Pathol. 2009, 217, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [PubMed]
- Zenzmaier, C.; Untergasser, G.; Berger, P. Aging of the prostate epithelial stem/progenitor cell. Exp. Gerontol. 2008, 43, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Yuan, J.; Wills, M.; Kasper, S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007, 67, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Maitland, N.J.; Bryce, S.D.; Stower, M.J.; Collins, A.T. Prostate cancer stem cells: A target for new therapies. Ernst Schering Found. Symp. Proc. 2006, 5, 155–179. [Google Scholar] [PubMed]
- Maitland, N.J.; Collins, A.T. Prostate cancer stem cells: A new target for therapy. J. Clin. Oncol. 2008, 26, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Dunning, N.L.; Laversin, S.A.; Miles, A.K.; Rees, R.C. Immunotherapy of prostate cancer: Should we be targeting stem cells and EMT? Cancer Immunol. Immunother. 2011, 60, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, F.; Du, X.; Lai, D.; Zhao, Y.; Huang, Q.; Jiang, L.; Huang, W.; Cheng, W.; Liu, Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem. 2010, 340, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Johansson, S.L.; Vankatraman, G.; Moore, E.; Henichart, J.P.; Depreux, P.; Lin, M.F.; Batra, S.K. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol. Cancer Ther. 2007, 6, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Johansson, S.L.; Henichart, J.P.; Depreux, P.; Batra, S.K. Cytotoxic effects induced by docetaxel, gefitinib, and cyclopamine on side population and nonside population cell fractions from human invasive prostate cancer cells. Mol. Cancer Ther. 2010, 9, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Weissman, I.L. Pten, tumorigenesis, and stem cell self-renewal. Cell 2006, 125, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Wu, H. Pten, stem cells, and cancer stem cells. J. Biol. Chem. 2009, 284, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Korsten, H.; Ziel-van der Made, A.; Ma, X.; van der Kwast, T.; Trapman, J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS ONE 2009, 4, e5662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kruithof-de Julio, M.; Economides, K.D.; Walker, D.; Yu, H.; Halili, M.V.; Hu, Y.P.; Price, S.M.; Abate-Shen, C.; Shen, M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009, 461, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.A.; Toivanen, R.; Bergren, S.K.; Chambon, P.; Shen, M.M. Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep. 2014, 8, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Sarkar, F.H. Prostate cancer stem cells: Molecular characterization for targeted therapy. Asian J. Androl. 2012, 14, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park) 2014, 28, 1101–1107, 1110. [Google Scholar] [PubMed]
- Sharifi, N.; Kawasaki, B.T.; Hurt, E.M.; Farrar, W.L. Stem cells in prostate cancer: Resolving the castrate-resistant conundrum and implications for hormonal therapy. Cancer Biol. Ther. 2006, 5, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Motiani, K.; Giridhar, P.V.; Kasper, S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp. Biol. Med. (Maywood) 2013, 238, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shiozawa, Y.; Taichman, R.S.; McCauley, L.K.; Pienta, K.; Keller, E. Prostate cancer and parasitism of the bone hematopoietic stem cell niche. Crit. Rev. Eukaryot. Gene Expr. 2012, 22, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Mateo, F.; Meca-Cortés, O.; Celià-Terrassa, T.; Fernández, Y.; Abasolo, I.; Sánchez-Cid, L.; Bermudo, R.; Sagasta, A.; Rodríguez-Carunchio, L.; Pons, M.; et al. SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol. Cancer 2014, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, J.; Zhang, Z.; Zhou, W.; Wang, A.J.; Heddleston, J.M.; Pinna, C.M.; Hubaud, A.; Stadler, B.; Choi, M.; Bar, M.; et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011, 71, 4640–4652. [Google Scholar] [CrossRef] [PubMed]
- Ravenna, L.; Principessa, L.; Verdina, A.; Salvatori, L.; Russo, M.A.; Petrangeli, E. Distinct phenotypes of human prostate cancer cells associate with different adaptation to hypoxia and pro-inflammatory gene expression. PLoS ONE 2014, 9, e96250. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Bae, K.; Siemann, D.W. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, D.; Liu, J.; Axcrona, K.; Kvalheim, G.; Stokke, T.; Nesland, J.M.; Suo, Z. Prostate cancer cell lines under hypoxia exhibit greater stem-like properties. PLoS ONE 2011, 6, e29170. [Google Scholar] [CrossRef] [PubMed]
- Weckermann, D.; Müller, P.; Wawroschek, F.; Harzmann, R.; Riethmüller, G.; Schlimok, G. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: Detection and prognostic value. J. Urol. 2001, 166, 699–703. [Google Scholar] [CrossRef]
- Rahim, F.; Hajizamani, S.; Mortaz, E.; Ahmadzadeh, A.; Shahjahani, M.; Shahrabi, S.; Saki, N. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ok Lee, S.; Liang, L.; Huang, C.K.; Li, L.; Wen, S.; Chang, C. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene 2014, 33, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Xin, L.; Morim, A.; Lawson, D.; Witte, O.; Wu, H. Lin−Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009, 69, 8555–8562. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, D.; Röper, K.; Fargeas, C.A.; Joester, A.; Huttner, W.B. Prominin: A story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2001, 2, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90, 5002–5012. [Google Scholar] [PubMed]
- Bauer, N.; Fonseca, A.V.; Florek, M.; Freund, D.; Jászai, J.; Bornhäuser, M.; Fargeas, C.A.; Corbeil, D. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs 2008, 188, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Karthaus, W.L.; Dalrymple, S.; Meeker, A.; DeMarzo, A.M.; Isaacs, J.T. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008, 68, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Rentala, S.; Yalavarthy, P.D.; Mangamoori, L.N. α1 and β1 integrins enhance the homing and differentiation of cultured prostate cancer stem cells. Asian J. Androl. 2010, 12, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, C. Alternative RNA splicing and cancer. Wiley Interdiscip. Rev. RNA 2013, 4, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Hirschfeld, M.; Jaeger, M.; Zur Hausen, A.; Braicu, I.; Sehouli, J.; Gitsch, G.; Stickeler, E. Alterations in expression pattern of splicing factors in epithelial ovarian cancer and its clinical impact. Int. J. Gynecol. Cancer 2013, 23, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The role of CD44 in disease pathophysiology and targeted treatment. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Liu, T.; Delgadillo, L.F.; Cuckler, C.M.; Tees, D.F.; Benencia, F.; Goetz, D.J.; Burdick, M.M. CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am. J. Physiol. Cell Physiol. 2015, 308, C68–C78. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Karikoski, M.; Elima, K.; Rantakari, P.; Jalkanen, S. CD44 binds to macrophage mannose receptor on lymphatic endothelium and supports lymphocyte migration via afferent lymphatics. Circ. Res. 2013, 112, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Hurt, E.M.; Mathews, L.A.; Zhang, X.; Duhagon, M.A.; Mistree, T.; Thomas, S.B.; Farrar, W.L. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 2009, 26, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.P.; He, L.; Kapoor, A.; Cutz, J.C.; Tang, D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim. Biophys. Acta 2011, 1813, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Vis, A.N.; Schröder, F.H. Key targets of hormonal treatment of prostate cancer. Part 1: The androgen receptor and steroidogenic pathways. BJU Int. 2009, 104, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Semenas, J.; Allegrucci, C.; Boorjian, S.A.; Mongan, N.P.; Persson, J.L. Overcoming drug resistance and treating advanced prostate cancer. Curr. Drug Targets 2012, 13, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Cozzi, P.; Hao, J.; Duan, W.; Graham, P.; Kearsley, J.; Li, Y. Cancer stem cells in prostate cancer chemoresistance. Curr. Cancer Drug Targets 2014, 14, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Davies, A.; Ketola, K.; Zoubeidi, A. Regulation of tumor cell plasticity by the androgen receptor in prostate cancer. Endocr. Relat. Cancer 2015, 22, R165–R182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, M.; Li, L.; Wu, D.; Wu, K.; Li, X.; Zhu, G.; Dang, Q.; Wang, X.; Hsieh, J.T.; et al. Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties. J. Cancer Res. Clin. Oncol. 2012, 138, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Uzgare, A.; Litvinov, I.; Denmeade, S.R.; Isaacs, J.T. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr. Relat. Cancer 2006, 13, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Njar, V.C.; Brodie, A.M. Discovery and development of galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J. Med. Chem. 2015, 58, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Chen, M.; Penning, T.M. AKR1C3 as a target in castrate resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2013, 137, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Fujimoto, N.; Naito, S. Androgen receptor cofactors in prostate cancer: Potential therapeutic targets of castration-resistant prostate cancer. Curr. Cancer Drug Targets 2011, 11, 870–881. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.T.; Iwai, A.; Feau, C.; Garcia, Y.; Balsiger, H.A.; Storer, C.L.; Suro, R.M.; Garza, K.M.; Lee, S.; Kim, Y.S.; et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11878–11883. [Google Scholar] [CrossRef] [PubMed]
- Kregel, S.; Kiriluk, K.J.; Rosen, A.M.; Cai, Y.; Reyes, E.E.; Otto, K.B.; Tom, W.; Paner, G.P.; Szmulewitz, R.Z.; Vander Griend, D.J. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS ONE 2013, 8, e53701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hwang, T.H.; Oseth, L.A.; Hauge, A.; Vessella, R.L.; Schmechel, S.C.; Hirsch, B.; Beckman, K.B.; Silverstein, K.A.; Dehm, S.M. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene 2012, 31, 4759–4767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Morrissey, C.; Sun, S.; Ketchandji, M.; Nelson, P.S.; True, L.D.; Vakar-Lopez, F.; Vessella, R.L.; Plymate, S.R. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE 2011, 6, e27970. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Van der Steen, T.; Tindall, D.J. Are androgen receptor variants a substitute for the full-length receptor? Nat. Rev. Urol. 2015, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Luo, J. Decoding the androgen receptor splice variants. Transl. Androl. Urol. 2013, 2, 178–186. [Google Scholar] [PubMed]
- Sun, Y.; Wang, B.E.; Leong, K.G.; Yue, P.; Li, L.; Jhunjhunwala, S.; Chen, D.; Seo, K.; Modrusan, Z.; Gao, W.Q.; et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: Implications for androgen-deprivation therapy. Cancer Res. 2012, 72, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Sethi, S.; Li, Y.; Chen, W.; Sakr, W.A.; Heath, E.; Sarkar, F.H. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015, 75, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Wang, G.; Struhl, K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. USA 2011, 108, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011, 71, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Klotz, L.H.; Venkateswaran, V. Metformin and prostate cancer stem cells: A novel therapeutic target. Prostate Cancer Prostatic Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Metformin and cancer. Rev. Diabet. Stud. 2013, 10, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Lin, S.H.; Tang, D.G.; Parikh, K.; Lee, M.H.; Yeung, S.C.; Tu, S.M. Maintenance therapy containing Metformin and/or Zyflamend for advanced prostate cancer: A case series. Case Rep. Oncol. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wicha, M.S.; Schwartz, S.J.; Sun, D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J. Nutr. Biochem. 2011, 22, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Ninfali, P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Gaigneaux, A.; Chateauvieux, S.; Billing, A.M.; Planchon, S.; Fack, F.; Renaut, J.; Mack, F.; Muller, C.P.; Dicato, M.; et al. Identification of differentially expressed proteins in curcumin-treated prostate cancer cell lines. OMICS 2012, 16, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Botchkina, G.I.; Zuniga, E.S.; Rowehl, R.H.; Park, R.; Bhalla, R.; Bialkowska, A.B.; Johnson, F.; Golub, L.M.; Zhang, Y.; Ojima, I.; et al. Prostate cancer stem cell-targeted efficacy of a new-generation taxoid, SBT-1214 and novel polyenolic zinc-binding curcuminoid, CMC2.24. PLoS ONE 2013, 8, e69884. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, D.; Król, W.; Szliszka, E. Prostate Cancer Stem Cells: Research Advances. Int. J. Mol. Sci. 2015, 16, 27433-27449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126036
Jaworska D, Król W, Szliszka E. Prostate Cancer Stem Cells: Research Advances. International Journal of Molecular Sciences. 2015; 16(11):27433-27449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126036
Chicago/Turabian StyleJaworska, Dagmara, Wojciech Król, and Ewelina Szliszka. 2015. "Prostate Cancer Stem Cells: Research Advances" International Journal of Molecular Sciences 16, no. 11: 27433-27449. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126036