Leptin Genes in Blunt Snout Bream: Cloning, Phylogeny and Expression Correlated to Gonads Development
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of Three Genes


| Target Gene | Leptin | LeptinR | Leprotl1 |
|---|---|---|---|
| cDNA length | 953 | 3432 | 1676 |
| 5’-Untranslated Region (UTR) | 81 | 25 | 111 |
| Open Reading Frame (ORF) | 514 | 3249 | 396 |
| ORF Encoding aa | 168 | 1082 | 131 |
| 3’-UTR | 371 | 158 | 1169 |
| GeneBank No. | KJ 193854 | KJ 193855 | KJ 193853 |
| CpG Island | 0 | 4 | 1 |
| Transmembrane Domain (TMD) | 0 | 1 | 4 |
| Signal Peptide | 1 | 1 | 0 |
| Fibronectin type-III domain | 0 | 3 | 0 |
| Genome Length (bp) | 1836 | 28,528 | 5480 |
| Exon | 3 | 15 | 4 |
| GeneBank No. | KP 269244 | KP 269245 | KP 269246 |
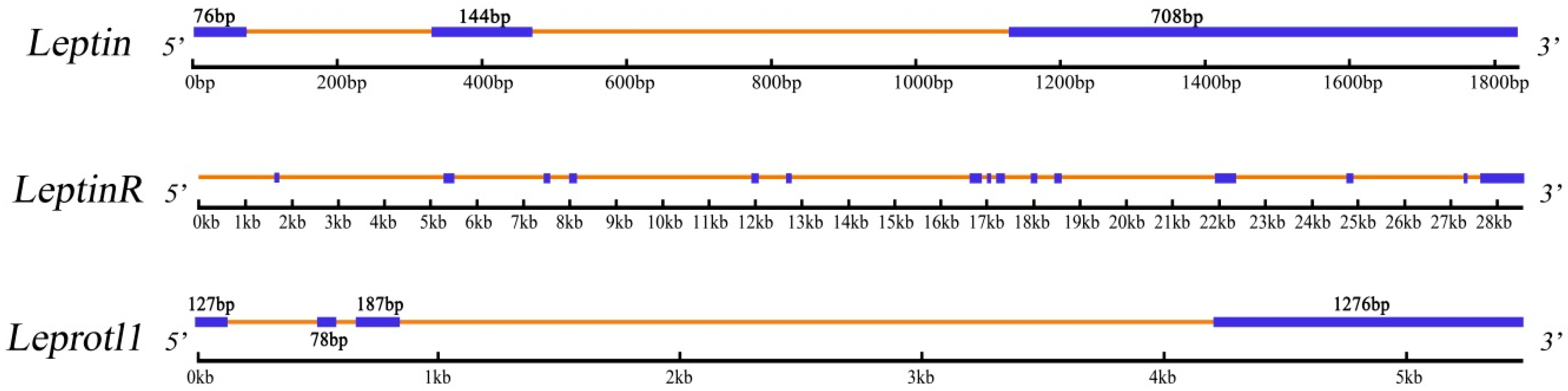
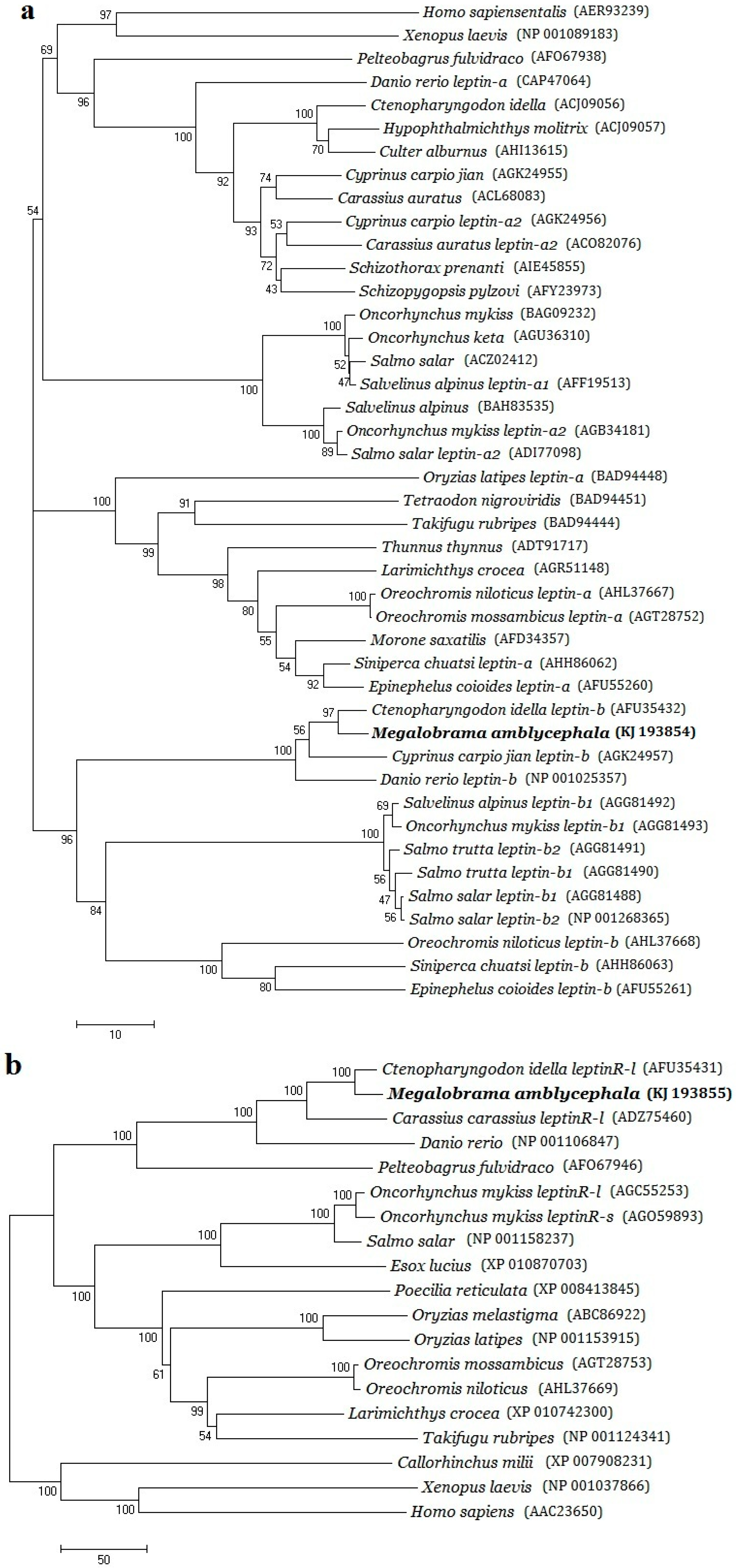
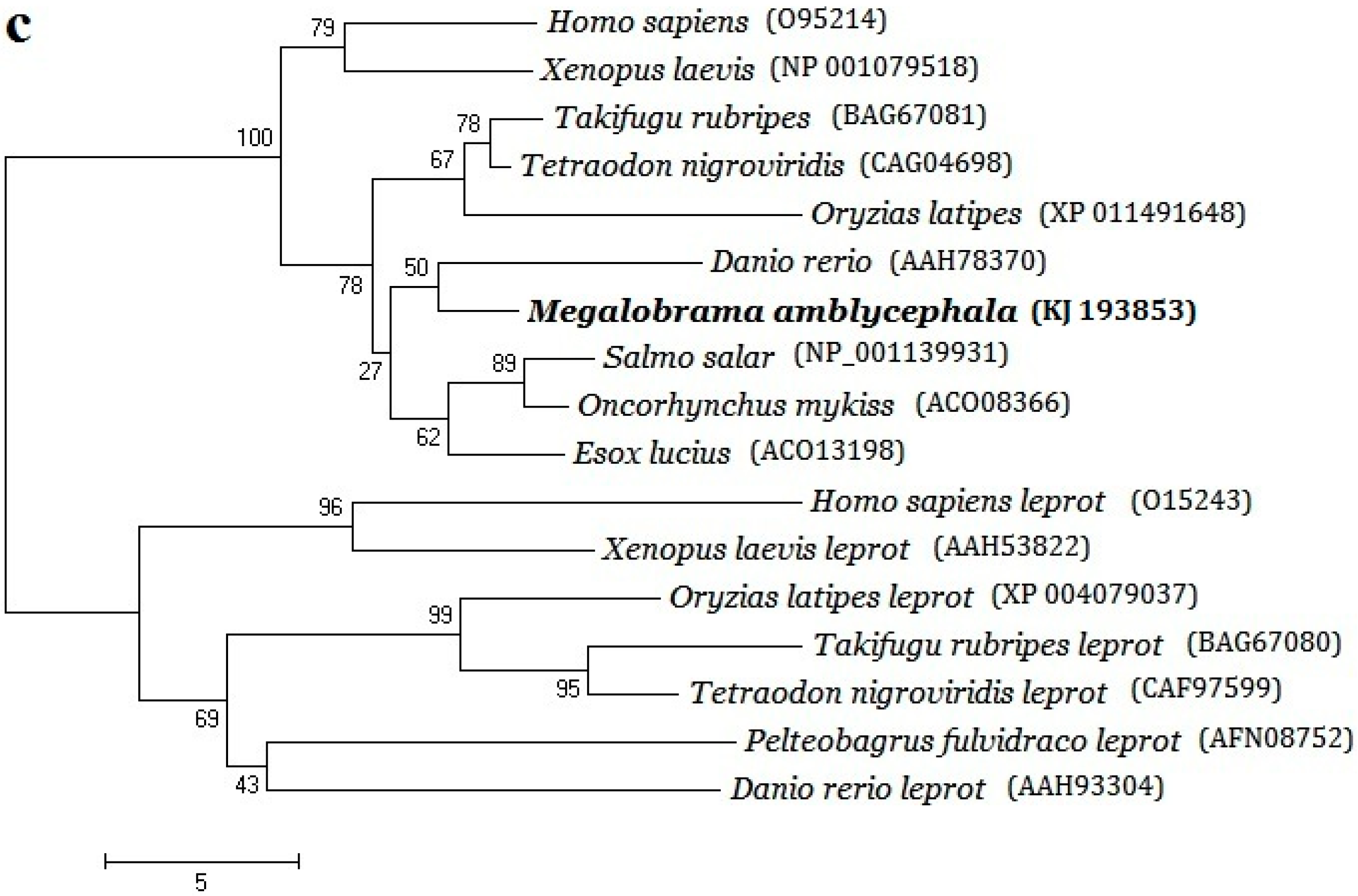
2.2. Phylogenetic Evolution Analysis
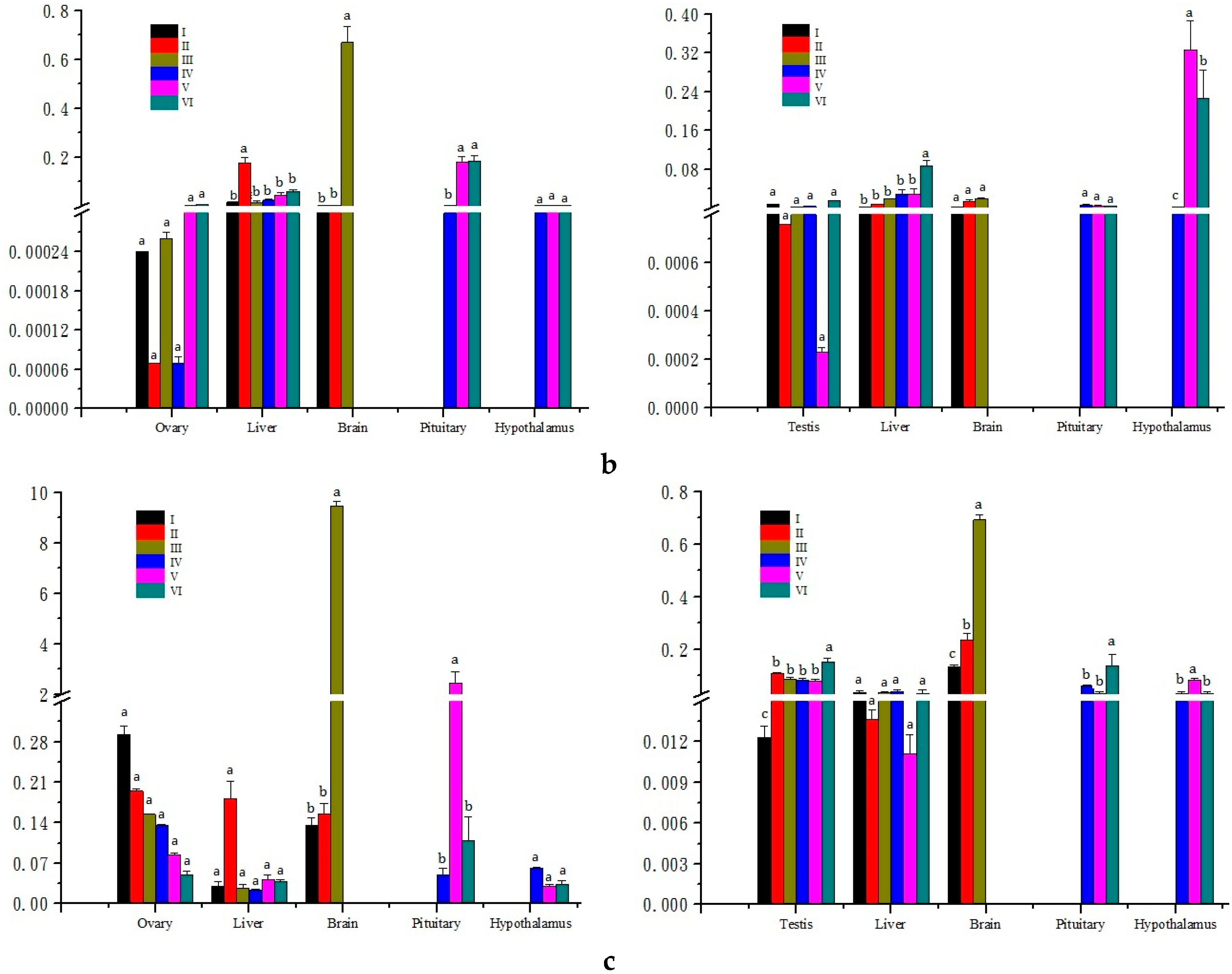
2.3. Expression Analysis in Different Developmental Stages of Gonads

2.4. Correlation Analysis of Three Genes
| Target Gene | Leptin | Leptinr | Leprotl1 |
|---|---|---|---|
| leptin | – | 0.752 ** | 0.751 ** |
| leptinR | 0.000 | – | 0.799 ** |
| leprotl1 | 0.000 | 0.000 | – |
3. Discussion
3.1. Structural Characterizations and Evolution Analysis of Three Genes
3.2. Analysis of Genes Exons and Introns
3.3. Expression Analysis of Three Genes
3.4. Correlation Analysis of Three Genes
4. Experimental Section
4.1. Experimental Animals
4.2. Molecular Cloning
| Target Gene | Primer Name | Primer Sequence (5’–3’) |
|---|---|---|
| Internal Control Primers for qRT-PCR | ||
| β-actin primers | β-actin-F | CGGACAGGTCATCACCATTG |
| β-actin-R | CGCAAGACTCCATACCCAAGA | |
| 18S primers | 18S-F | CGGAGGTTCGAAGACGATCA |
| 18S-R | GGGTCGGCATCGTTTACG | |
| Quantitative Primers of Three Genes | ||
| leptin primers | leptin-F | CAGTTGAGATTGCGAGTGCC |
| leptin-R | GTTGGAGGTAACGGGGAAGG | |
| leptinR primers | leptinR-F | TAGACGAACCAGGGTTTGATA |
| leptinR-R | ATTCTTGCTCTGGCAGGTAA | |
| leprotl1 primers | leprotl1-F | CAGTTGGCAGCAGTGGTGAAG |
| leprotl1-R | CATCTATCAATGGGCGGCAGT | |
| Gene Specific Primers for RACE | ||
| leptin specific primers for 3’ RACE | 3’-1 | TTTTCCCTCTCAATGGCAGCCCTGGG |
| 3’-2 | GTACCTGGAAAAGCTTTGCCTGAA | |
| leptin specific primers for 5’ RACE | 5’-1 | CACATCAACACACCAGAGAAAGTC |
| 5’-2 | CCAACATACTACCAGCTTC | |
| leptinR specific primers for 3’ RACE | 3’-1 | TTTTCCCTCTCAATGGCAGCCCTGG |
| 3’-2 | GGTACCTGGAAAAGCTTTGCCTGAA | |
| leptinR specific primers for 5’ RACE | 5’-1 | CACATCAACACACCAGAGAAAG |
| 5’-2 | TCCCAACATACTACCAGCTTC | |
| leprotl1 specific primers for 3’ RACE | 3’-1 | GCATTGTGAGGTTTCCAGATTTCCCA |
| 3’-2 | ATCCCCCCGAAACTAATGAAGAAGCAG | |
| leprotl1 specific primers for 5’ RACE | 5’-1 | GTGTGTTTATGAATCTACGCA |
| 5’-2 | CGAAAATGGCATTTATTTAAAAAAAGAAC | |
| Introns Primers for Genomic Sequence | ||
| leptin-Intron | F-primer | ATCATGGCCCGAACTACCATC |
| R-primer | TGTCCATGTTCAGGCAAAGC | |
| leptinR-Intron-1 | F-primer | TTCATTGCCGTTTCACAAGG |
| R-primer | AGGGCAAGTGATTTACTAGAGG | |
| leptinR-Intron-2 | F-primer | TTGTGAGCTGCCCTTTGCTC |
| R-primer | ATCTTCGGTAACAGTGCGGT | |
| leptinR-Intron-3 | F-primer | ATCTGCGAATGGGACTACCG |
| R-primer | TTCAGAGGCACCCATGAACG | |
| leptinR-Intron-4 | F-primer | AGGTCAGAGGTCGTTCATGG |
| R-primer | AGCTTCTTCAGTGCTCTCCG | |
| leptinR-Intron-5 | F-primer | GTGCGAGTAAAGCTGTGTGG |
| R-primer | TGCCTTCTGTGATTGAGCACT | |
| leptinR-Intron-6 | F-primer | TGGAACCACAGGCAGAGATT |
| R-primer | CACTGACAGGAACGCAATGAT | |
| leptinR-Intron-7 | F-primer | ATTGCGTTCCTGTCAGTGGT |
| R-primer | GAAGTCCATTCCTTTTGCCCAG | |
| leptinR-Intron-8 | F-primer | CCTGGGCAAAAGGAATGGAC |
| R-primer | CTGGAATGGCGAGGATTGGT | |
| leprotl1-Intron-1 | F-primer | TCGCGAGGAGTTTCTTTAGCTT |
| R-primer | TATAAACGGGCAGAGCGCAG | |
| leprotl1-Intron-2 | F-primer | AGTCTGTCGTTTGGAGGAGC |
| R-primer | CAATCCCCGTGGTCAGGAAT | |
| leprotl1-Intron-3 | F-primer | ACTCGGCTAGTAATGCCTGC |
| R-primer | TGAAGACGGATGAGACGCTG | |
4.3. Sequence Analysis
4.4. Quantitative Real-Time PCR Analysis of Tissue Expression
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Proenca, P.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Boswell, T.; Dunn, I.C.; Wilson, P.W.; Joseph, N.; Burt, D.W.; Sharp, P.J. Identificationofanon mammalian leptin-like gene: Characterization and expression in the tiger salamander (Ambystoma tigrinum). Gen. Comp. Endocrinol. 2006, 146, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Crespi, E.J.; Denver, R.J. Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc. Natl. Acad. Sci. Biol. 2006, 103, 10092–10097. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish Takifugu rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Pedro, N.D.; Alvarez, R.M.; Delgado, M.J. Acute and chronic leptin-reduces food intake and body weight in goldfish (Carassius auratus). Endocrinology 2006, 188, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Huising, M.O.; Geven, E.J.W.; Kruiswijk, C.P.; Nabuurs, S.B.; Stolte, E.H.; Spanings, F.A.T.; Kemenade, B.M.L.V.; Flik, G. Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology 2006, 147, 5786–5797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashita, K.; Uji, S.; Yamamoto, T.; Rønnestad, I.; Kurokawa, T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss). Biochem. Mol. Biol. 2008, 150, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Bernier, N.J.; Nabuurs, S.B.; Flik, G.; Huising, M.O. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. Endocrinology 2009, 201, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, G.G.; Liang, X.F.; Xie, Q.; Li, G.; Yu, Y.; Lai, K. Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen. Comp. Endocrinol. 2010, 166, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, Z.; Qing, L.Z.; Jia, L.Z.; Xiao, Y.T.; Qi, L.C.; Yu, C.L.; Rong, H.L. Characterization and tissue distribution of leptin, leptin receptor and leptin receptor overlapping transcript genes in yellow catfish Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 2013, 182, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Murashita, K.; Suzuki, T.; Uji, S. Genomic characterization and tissue distribution of leptinR and leptinR overlapping transcript genes in the pufferfish Takifugu rubripes. Gen. Comp. Endocr. 2008, 158, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Murashita, K. Genomic characterization of multiple Leptin genes and a leptinR gene in the Japanese medaka Oryzias latipes. Gen. Comp. Endocrinol. 2009, 161, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.B.; Xue, J.L.; Wu, L.Y.; Jiang, W.; Hu, P.N.; Zhu, J. The detection of leptinR isoforms in crucian carp gill and the influence of fasting and hypoxia on their expression. Domest. Anim. Endocrinol. 2011, 4, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, B.; Akerblom, I.; Strosberg, A.D. The leptin receptor promoter controls expression of a second distinct protein. Nucleic Acids Res. 1997, 25, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Won, E.T.; Baltzegar, D.A.; Picha, M.E.; Borski, R.J. Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): Control of feeding and regulation by nutritional state. Gen. Comp. Endocrinol. 2012, 178, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.D.; Qin, L.; Mason, D.K.; Brian, B.; Richard, L.L. Leptin expression affects metabolic rate in zebrafish embryos (D. rerio). Front. Physiol. 2013, 4. [Google Scholar] [CrossRef]
- Trombley, S.; Schmitz, M. Leptin in fish: Possible role in sexual maturation in male Atlantic salmon. Fish Physiol. Biochem. 2013, 39, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Denver, R.J.; Bonett, R.M.; Boorse, G.C. Evolution of leptin structure and function. Neuroendocrinology 2011, 94, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Peyon, P.; Zanuy, S.; Carillo, M. Actions of leptin on in vitro luteinizing hormone release in the European sea bass (Dicentrarchus labrax). Biol. Reprod. 2001, 65, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Weil, C.; Le, B.P.Y.; Sabin, N.; Le, G.F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 2003, 130, 2–12. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Yasmeen, G.; Li, S.; Wang, W.M. Cloning, identification and accurate normalization expression analysis of PPARα gene by GeNorm in Megalobrama amblycephala. Fish Shellfish Immun. 2011, 31, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.F.; Gao, Z.X.; Luo, W.; Tong, J.G.; Wang, W.M. Observation of gonad at different development stages and expression analysis of Kiss2/Kiss2r genes in Megalobrama amblycephala. South China Fish. Sci. 2013, 9, 44–50. [Google Scholar]
- Prokop, J.W.; Duff, R.J.; Ball, H.C.; Copeland, D.L.; Londraville, R.L. Leptin and leptin receptor analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptide 2012, 38, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Kryukov, K.; Clemente, J.C.; Komiyama, T.; Suzuki, Y.; Imanishi, T. The evolutionary relationship between gene duplication and alternative splicing. Gene 2008, 427, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Anna, R.A.; Sigurd, O.S.; Tom, O.N.; Raja, M.R.; Ivar, R. Molecular cloning and genomic characterization of novel leptin-like genes in salmonids provide new insight into the evolution of the Leptin gene family. Gen. Comp. Endocrinol. 2013, 187, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Xu, F.L.; Ling, L.; Wei, H.; Dan, S.; Ya, X.T. Gene structure and expression of leptin in Chinese perch. Gen. Comp. Endocrinol. 2013, 194, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Liu, X.L.; Wang, W.M.; Tong, J.G.; Luo, W.; Zhang, J.; Gao, Z.X. Characterization of GHRs, IGFs and MSTNs, and analysis of their expression relationships in blunt snout bream, Megalobrama amblycephala. Gene 2014, 535, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Louis, A.T. The Leptin Receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, Y.P.; Yu, L. Natural selection and adaptive evolution of leptin. Chin. Sci. Bull. 2013, 58, 2104–2112. [Google Scholar] [CrossRef]
- Yu, L.; Jin, W.; Zhang, X.; Wang, D.; Zheng, J.S.; Yang, G.; Xu, S.X. Evidence for positive selection on the Leptin gene in cetacea and pinnipedia. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.M.; He, Y.; Chen, H.; Quon, M.J.; Reitman, M. Regulation of leptin promoter function by SP1, C/EBP, and a novel factor. Endocrinology 1998, 139, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Donald, L.C.; Robert, J.D.; Qin, L.; Jeremy, P.; Richard, L.L. Leptin in teleost fishes: An argument for comparative study. Front. Physiol. 2011, 2. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, X.F.; Li, G.G.; Wang, L.; Li, G.Z. Molecular cloning and analysis of leptin gene in grass carp (Ctenopharyngodon idellus) and silver carp (Hypophthalmichthys molitrix). Zool. Res. 2009, 30, 480–486. [Google Scholar]
- Mercer, J.G.; Moar, K.M.; Hoggard, N.; Strosberg, A.D.; Froguel, P.; Bailleul, B. B219/OB-R 5′-UTR and leptin receptor gene-related protein gene expression in mouse brain and placenta: Tissue-specific leptin receptor promoter activity. J. Neuroendocrinol. 2000, 12, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Carol, F.E.; Darshana, P. Leptin signaling and circuits in puberty and fertility. Cell. Mol. Life Sci. 2013, 70, 841–862. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Frank, C.; Luc, J.M. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod. Biol. 2013, 13, 1–14. [Google Scholar] [PubMed]
- Dhirender, V.R.; Carol, F.E. Chemical identity of hypothalamic neurons engaged by leptin in reproductive control. Chem. Neuroanat. 2014, 61, 233–238. [Google Scholar]
- Stergios, M.; Jean, L.C.; Christos, S.M. Leptin and reproduction: A review. Fertil. Steril. 2002, 10, 433–444. [Google Scholar]
- Thierry, T.; Françoise, C.A.; Olivier, B.; Céline, C.; Réjane, P.; Sandrine, C.; Eric, B.; Yves, R.; Jean, P.S.; Bart, S.; et al. LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice. Clin. Investig. 2009, 199, 3830–3838. [Google Scholar]
- Chisada, S.; Kurokawa, T.; Murashita, K.; Rønnestad, I.; Taniguchi, Y.; Toyoda, A.; Sakaki, Y.; Takeda, S.; Yoshiura, Y. Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen. Comp. Endocrinol. 2014, 195, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.W. An excellent freshwater food fish, Megalobrama amblycephala, and its propagating and culturing. Acta Hydrobiol. Sin. 1975, 5, 293–312. [Google Scholar]
- Gao, Z.; Luo, W.; Liu, H.; Zeng, C.; Liu, X.; Yi, S.; Wang, W. Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS ONE 2012, 7, e42637. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Available online: http://0-www-Ncbi-Nlm-Nih-Gov.brum.beds.ac.uk/gorf/orfig.cgi (accessed on 14 February 2014).
- Signal 4.0 Server. Available online: http://www.cbs.dtu.dk/services/SignalP/ (accessed on 20 January 2014).
- Uniprot. Available online: http://www.uniprot.org/ (accessed on 20 January 2014).
- SMART. Available online: http://smart.embl- heidelberg.de/ (accessed on 23 January 2014).
- ExPASy. Available online: http://us.ex pasy.org/tools (accessed on 22 January 2014).
- TMHMM. Available online: http://www.cbs.dtu.dk/services/TMHMM-2.0/ (accessed on 2 May 2014).
- GeNorm. Available online: http://medgen.ugent.be/genorm/ (accessed on 10 December 2013).
- Zhang, H.; Chen, H.; Zhang, Y.; Li, S.; Lu, D.; Zhang, H.; Meng, Z.; Liu, X.; Lin, H. Molecular cloning, characterization and expression profiles of multiple leptin genes and leptinR genes in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2013, 181, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, J.L.; Thomas, D.S. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zeng, C.; Yi, S.; Wan, S.; Chen, B.; Gao, Z. Leptin Genes in Blunt Snout Bream: Cloning, Phylogeny and Expression Correlated to Gonads Development. Int. J. Mol. Sci. 2015, 16, 27609-27624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126044
Zhao H, Zeng C, Yi S, Wan S, Chen B, Gao Z. Leptin Genes in Blunt Snout Bream: Cloning, Phylogeny and Expression Correlated to Gonads Development. International Journal of Molecular Sciences. 2015; 16(11):27609-27624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126044
Chicago/Turabian StyleZhao, Honghao, Cong Zeng, Shaokui Yi, Shiming Wan, Boxiang Chen, and Zexia Gao. 2015. "Leptin Genes in Blunt Snout Bream: Cloning, Phylogeny and Expression Correlated to Gonads Development" International Journal of Molecular Sciences 16, no. 11: 27609-27624. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126044






