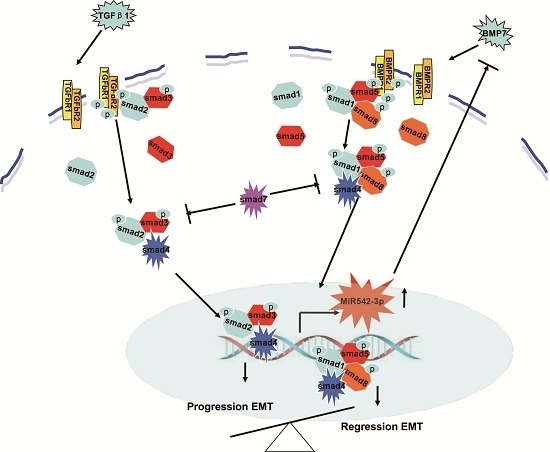
MiR542-3p Regulates the Epithelial-Mesenchymal Transition by Directly Targeting BMP7 in NRK52e
Abstract
:1. Introduction
2. Results
2.1. MiR542-3p Expression Was Up-Regulated in Fibrotic Kidney
2.2. Identification of TGFβ1 as a Positive Regulator of MiR542-3p Expression

2.3. MiR542-3p Induce Epithelial-to-Mesenchymal Transition (EMT)
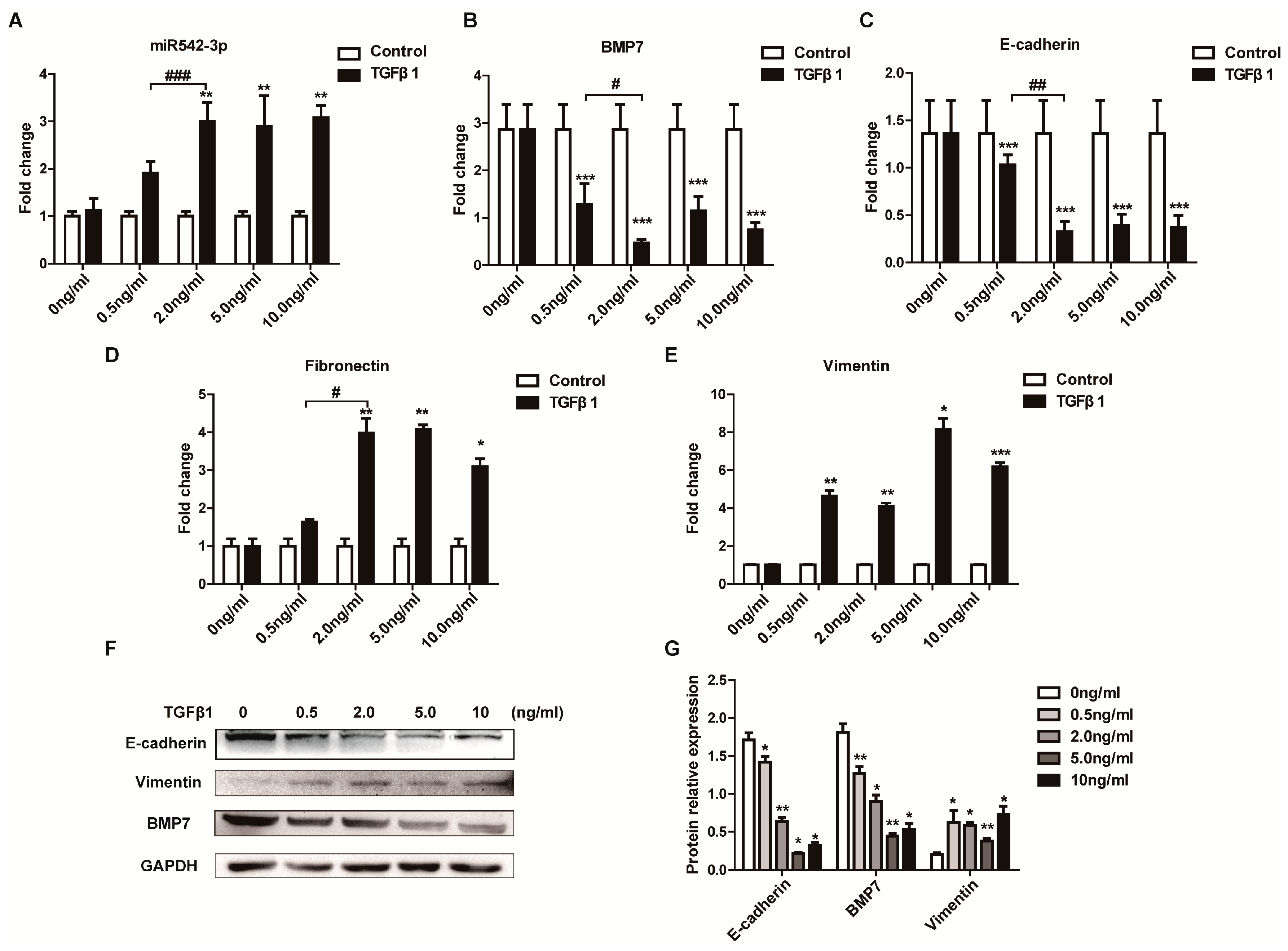
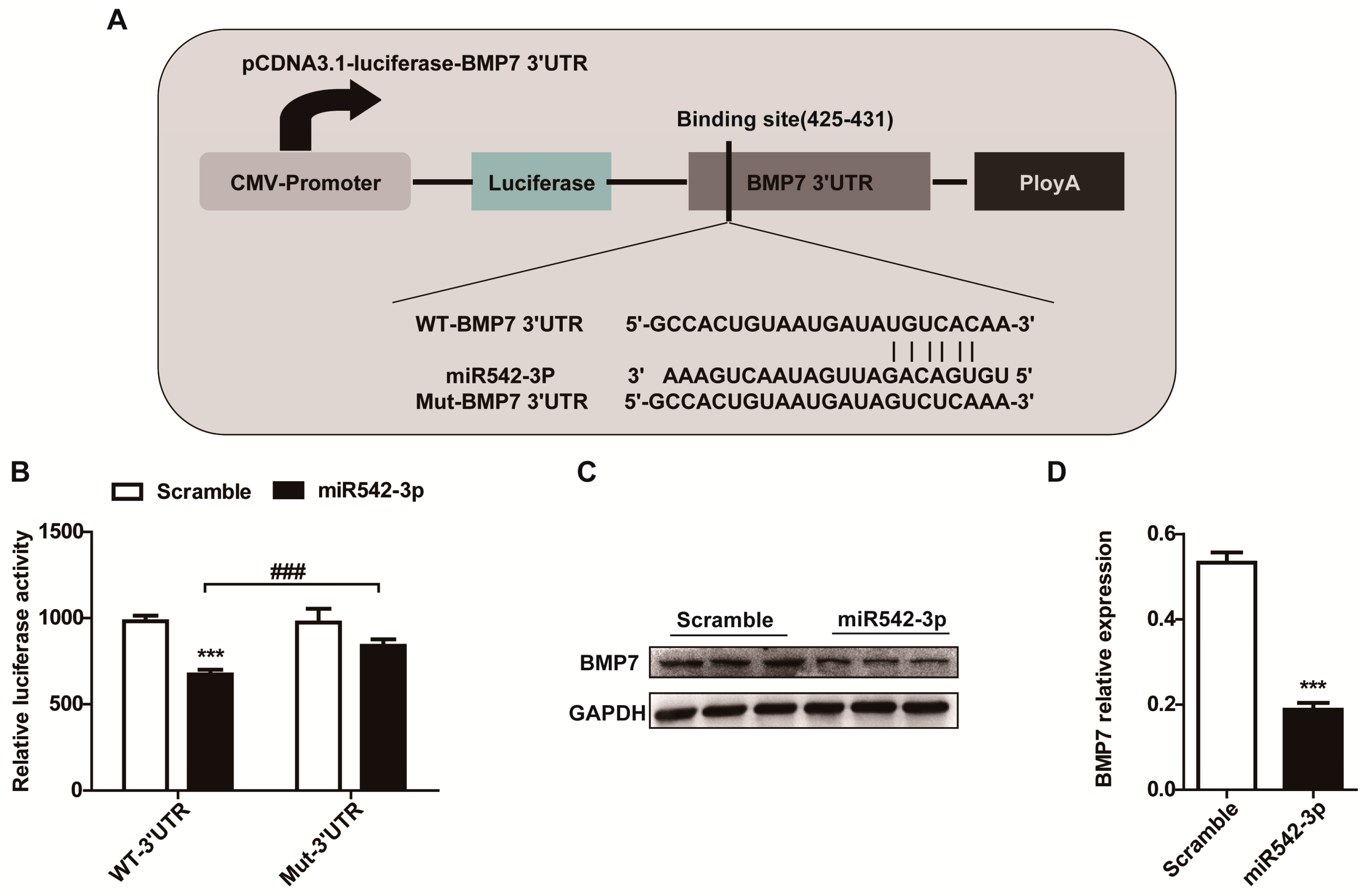
2.4. BMP7 Is a Direct Target of MiR542-3p
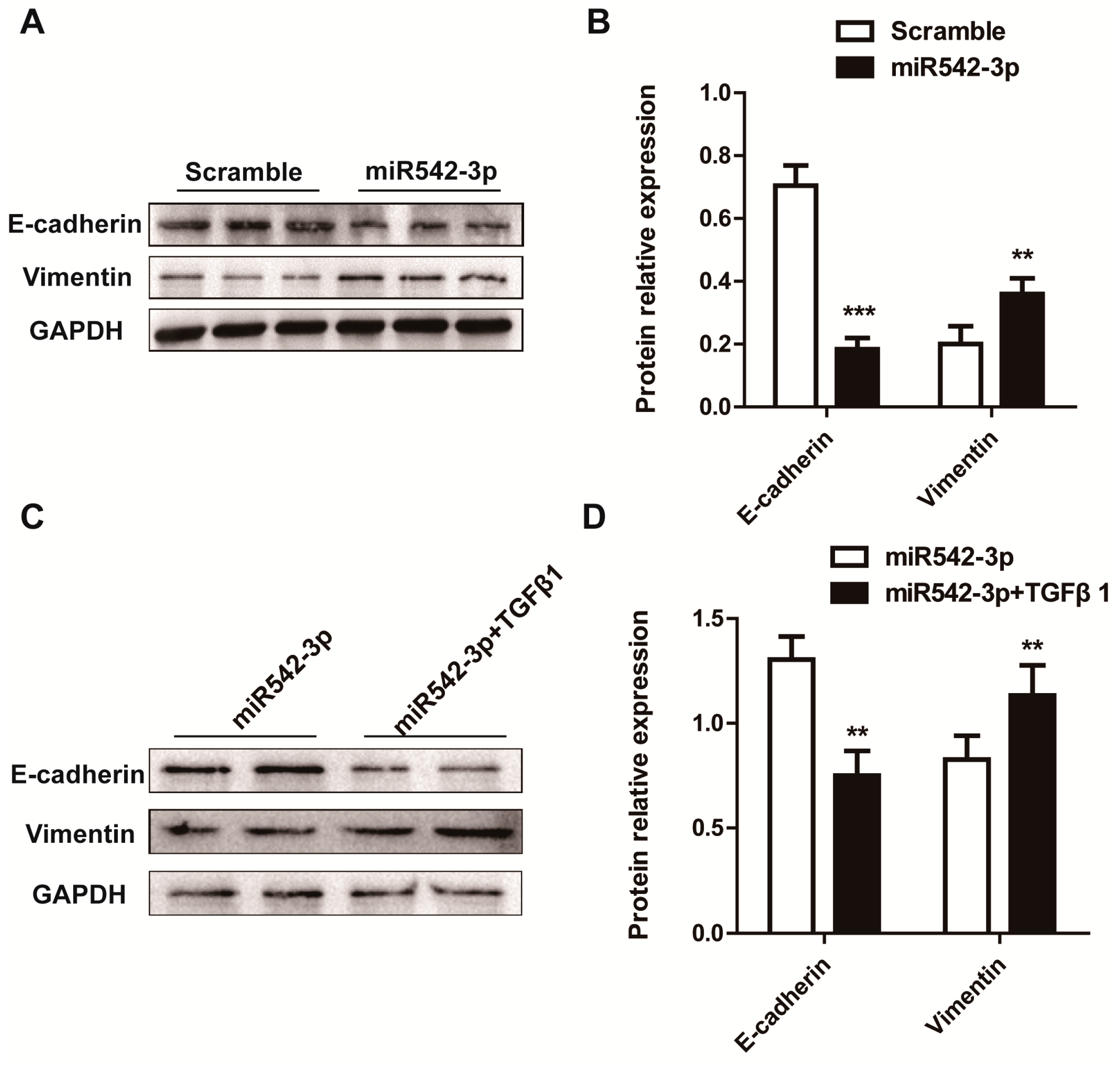
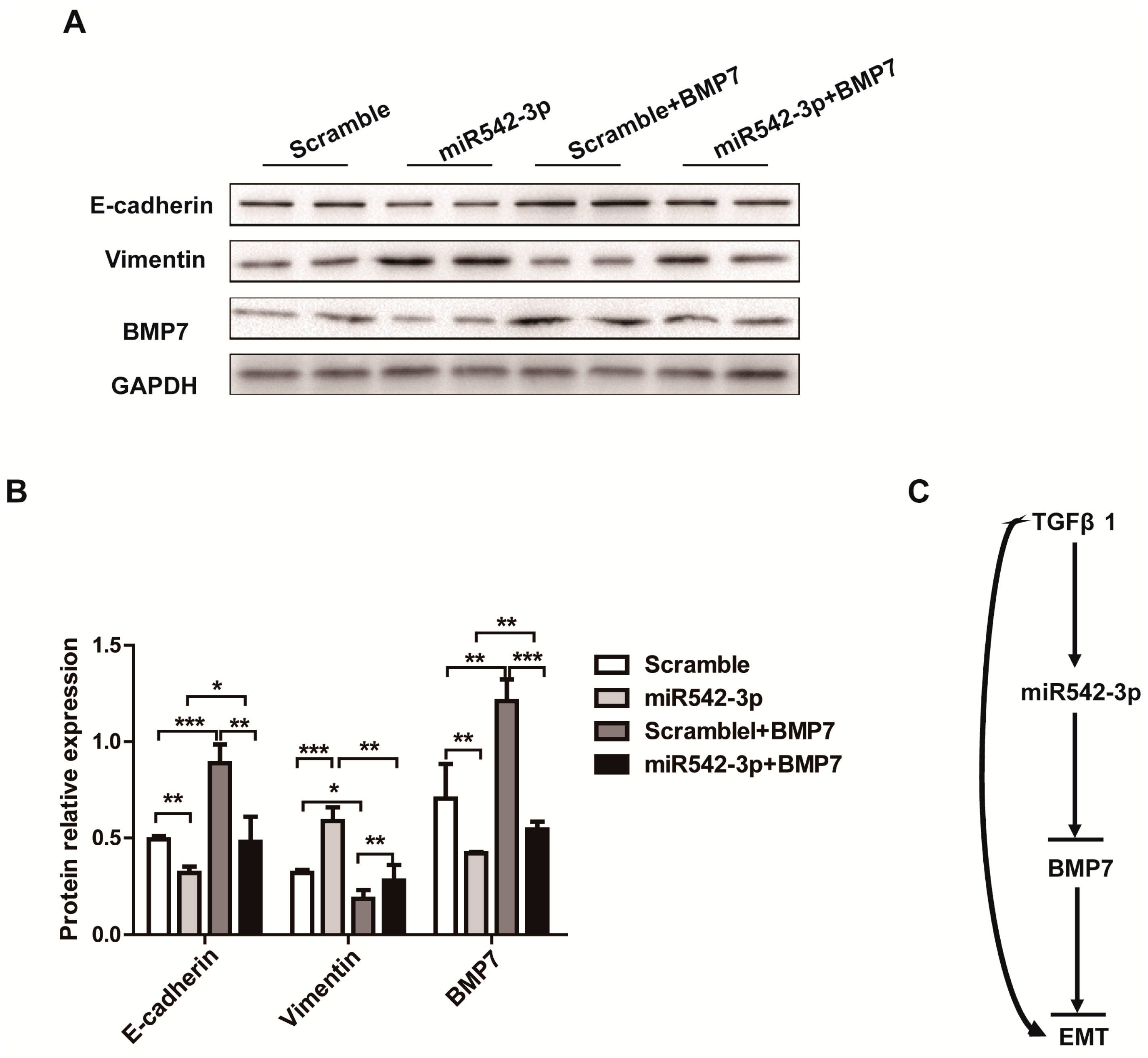
2.5. Overexpression of BMP7 Attenuates the MiR542-3p-Induced EMT
3. Discussion
4. Experimental Section
4.1. Obstructive Kidney Disease Model
4.2. Cell Culture and Transfection
4.3. RNA Isolation and Quantitative Real-Time PCR Analysis
| Gene Name | Sense | Antisense |
|---|---|---|
| BMP7 | CAGCCACCAGCAACCACT | GTCCATGCCGTCCAATCA |
| Ecadherin | GTCAACACCTACAACGCTGC | ACGTGCTTGGGTTGAAGACA |
| Vimentin | TGACCGCTTCGCCAACTA | CGCAACTCCCTCATCTCCT |
| Collagen I | AACTTTGCTTCCCAGATFTCCTATG | GCTTCCCCATCATCTCCATTCTTGC |
| a-SMA | GGGGTGATGGTGGGAATG | GGGGTGATGGTGGGAATG |
| Fibnectin | TGTGACCCAGACTTACGG | TGTAGGTGAACGGGAGAA |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| 18sRNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
4.4. Western Bolt Analysis
4.5. Dual Luciferase Reporter Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohle, A.; Christ, H.; Grund, K.E.; Mackensen, S. The role of the interstitium of the renal cortex in renal disease. Contrib. Nephrol. 1979, 16, 109–114. [Google Scholar] [PubMed]
- Borges, F.T.; Melo, S.A.; Ozdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H., 2nd; LeBleu, V.S.; Kalluri, R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, S.I.; Choi, M.E. Therapeutic targets for treating fibrotic kidney diseases. Transl. Res. J. Lab. Clin. Med. 2015, 165, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Lee, Y.J.; Park, S.H.; Lee, J.H.; Taub, M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2005, 288, F988–F996. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.; Nagase, S.; Ueda, A.; Oteki, T.; Takada, K.; Obara, M.; Inoue, M.; Yoh, K.; Hirayama, K.; Koyama, A. In vivo imaging of oxidative stress in ischemia-reperfusion renal injury using electron paramagnetic resonance. Am. J. Physiol. Ren. Physiol. 2005, 288, F597–F603. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Vaziri, N.D.; Herrera-Acosta, J.; Johnson, R.J. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. Am. J. Physiol. Ren. Physiol. 2004, 286, F606–F616. [Google Scholar] [CrossRef] [PubMed]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenes. Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, W.; Xu, J.; Shao, L.; Wang, Y. Effect of BMP7 on podocyte transdifferentiation and Smad7 expression induced by hyperglycemia. Clin. Nephrol. 2015, 84, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhou, X.; Zhou, X.; Pi, C.; Xu, R.; Wan, M.; Yang, J.; Zhou, Y.; Liu, C.; Sun, J.; et al. BMP7 and EREG Contribute to the Inductive Potential of Dental Mesenchyme. Sci. Rep. 2015, 5, 9903. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; He, S.; Zeng, N.; Ma, J.; Zhang, B.; Shi, B.; Jia, Z. BMP7 Gene involved in nonsyndromic orofacial clefts in Western han Chinese. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shao, Q.; Ji, D.; Li, F.; Chen, G. Mesenchymal stem cells mitigate cirrhosis through BMP7. Cell. Physiol. Biochem. 2015, 35, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Pauk, M.; Grgurevic, L.; Brkljacic, J.; Kufner, V.; Bordukalo-Niksic, T.; Grabusic, K.; Razdorov, G.; Rogic, D.; Zuvic, M.; Oppermann, H.; et al. Exogenous BMP7 corrects plasma iron overload and bone loss in Bmp6−/− mice. Int. Orthop. 2015, 39, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, N.; Bauer, T.; Modak, M.; Wagner, K.; Schuster, C.; Koffel, R.; Seyerl, M.; Stockl, J.; Elbe-Burger, A.; Graf, D.; et al. Identification of bone morphogenetic protein 7 (BMP7) as an instructive factor for human epidermal Langerhans cell differentiation. J. Exp. Med. 2013, 210, 2597–2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Asada, M.; Asada, N.; Nakamura, J.; Oguchi, A.; Higashi, A.Y.; Endo, S.; Robertson, E.; Kimura, T.; Kita, T.; et al. BMP7 maintains undifferentiated kidney progenitor population and determines nephron numbers at birth. PLoS ONE 2013, 8, e73554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Agarwal, P.; Thangjam, G.S.; Radhesh, R.; Rao, S.G.; Kondaiah, P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors 2011, 29, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.; Ito, M.; Makarenkova, H.P.; Faber, S.C.; Lang, R.A. BMP7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development 2004, 131, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Kalluri, R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr. Nephrol. 2008, 23, 1395–1938. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Dressler, G.R. BMP7 signaling in renal development and disease. Trends Mol. Med. 2005, 11, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, J.; Zhang, N.; Xu, X.; Li, R.; Yi, Y.; Fang, L.; Zhang, L.; Li, M.; Wu, J.; et al. MicroRNA-542-3p Suppresses Tumor Cell Invasion via Targeting AKT Pathway in Human Astrocytoma. J. Biol. Chem. 2015, 290, 24678–24688. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Si, Y.; Yang, Z.; Wang, Q.; Yuan, J.; Zhang, X. MicroRNA-542-3p suppresses cell growth of gastric cancer cells via targeting oncogene astrocyte-elevated gene-1. Med. Oncol. 2015, 32. [Google Scholar] [CrossRef] [PubMed]
- Althoff, K.; Lindner, S.; Odersky, A.; Mestdagh, P.; Beckers, A.; Karczewski, S.; Molenaar, J.J.; Bohrer, A.; Knauer, S.; Speleman, F.; et al. MiR-542-3p exerts tumor suppressive functions in neuroblastoma by downregulating Survivin. Int. J. Cancer 2015, 136, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.W.; Castella, M.; Huntsman, D.G.; Taniguchi, T. p53 is positively regulated by miR-542-3p. Cancer Res. 2014, 74, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Kureel, J.; Dixit, M.; Tyagi, A.M.; Mansoori, M.N.; Srivastava, K.; Raghuvanshi, A.; Maurya, R.; Trivedi, R.; Goel, A.; Singh, D. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis. 2014, 5, e1050. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Qi, F.; Jia, L.; Wang, S.; Song, N.; Guo, L.; Fu, Y.; Luo, Y. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J. Pathol. 2014, 232, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Bray, I.; Tivnan, A.; Bryan, K.; Foley, N.H.; Watters, K.M.; Tracey, L.; Davidoff, A.M.; Stallings, R.L. MicroRNA-542-5p as a novel tumor suppressor in neuroblastoma. Cancer Lett. 2011, 303, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Choi, Y.C.; Lee, S.; Jeong, Y.; Yoon, J.; Baek, K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010, 584, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gan, H.; Zhang, H.; Tang, W.; Sun, Y.; Tang, X.; Kong, D.; Zhou, J.; Wang, Y.; Zhu, Y. MicroRNA21 inhibits SMAD7 expression through a target sequence in the 3′ untranslated region and inhibits proliferation of renal tubular epithelial cells. Mol. Med. Rep. 2014, 10, 707–712. [Google Scholar] [PubMed]
- Wang, J.Y.; Gao, Y.B.; Zhang, N.; Zou, D.W.; Wang, P.; Zhu, Z.Y.; Li, J.Y.; Zhou, S.N.; Wang, S.C.; Wang, Y.Y.; et al. MiR-21 overexpression enhances TGF-beta1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol. Cell. Endocrinol. 2014, 392, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.B.; Jiang, Z.P. Role of miR-21 and its signaling pathways in renal diseases. J. Recept. Signal Transduct. 2014, 34, 335–337. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, C.; Li, J. MiR-21 is a critical therapeutic target for renal fibrosis. Cell Biochem. Biophys. 2014, 68, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.; Huang, X.R.; Meng, X.; Lan, H.Y. MiR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Herman-Edelstein, M.; Koh, P.; Burns, W.; Jandeleit-Dahm, K.; Watson, A.; Saleem, M.; Goodall, G.J.; Twigg, S.M.; Cooper, M.E.; et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 2010, 59, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Qi, F.; Jia, L.; Wang, S.; Wang, C.; Song, N.; Fu, Y.; Li, L.; Luo, Y. Tumor cell-secreted angiogenin induces angiogenic activity of endothelial cells by suppressing miR-542-3p. Cancer Lett. 2015, 368, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.H.; Gao, P.; Feng, H.; Qin, Z.X.; Li, J.B.; Huang, L. Down-regulation of mir-542-3p promotes neointimal formation in the aging rat. Vasc. Pharmacol. 2015, 72, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Oneyama, C.; Morii, E.; Okuzaki, D.; Takahashi, Y.; Ikeda, J.; Wakabayashi, N.; Akamatsu, H.; Tsujimoto, M.; Nishida, T.; Aozasa, K.; et al. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene 2012, 31, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Bo, Y.; Shen, W.; Tan, J.; Jia, Z.; Xu, C.; Li, F. Leonurine ameliorates kidney fibrosis via suppressing TGF-β and NF-κB signaling pathway in UUO mice. Int. Immunopharmacol. 2015, 25, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Choi, M.E. Regulation of autophagy by TGF-β: Emerging role in kidney fibrosis. Semin. Nephrol. 2014, 34, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, M. Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol. Dial. Transplant. 2012, 27, 3686–3691. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.E.; Ding, Y.; Kim, S.I. TGF-β signaling via TAK1 pathway: Role in kidney fibrosis. Semin. Nephrol. 2012, 32, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Meister, G. SMADs stimulate miRNA processing. Mol. Cell 2010, 39, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chung, A.C.; Chen, H.Y.; Meng, X.M.; Lan, H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S. The pathogenesis of tubulointerstitial disease and mechanisms of fibrosis. Curr. Top. Pathol. 1995, 88, 51–67. [Google Scholar] [PubMed]
- Iwano, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.A.; Zeisberg, M.; Strutz, F. The importance of tubulointerstitial damage in progressive renal disease. Nephrol. Dial. Transplant. 2000, 15 (Suppl. 6), 76–77. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Sharma, A.; Rodier, J.T.; Klibanov, A.M.; Rieger, F.G.; Mohan, R.R. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS ONE 2013, 8, e66434. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hirschberg, R. BMP7 antagonizes TGF-β-dependent fibrogenesis in mesangial cells. Am. J. Physiol. Ren. Physiol. 2003, 284, F1006–F1013. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhou, Y.; Yuan, Y.; Nie, F.; Peng, R.; Li, Q.; Lyu, Z.; Mao, Z.; Huang, L.; Zhou, L.; et al. MiR542-3p Regulates the Epithelial-Mesenchymal Transition by Directly Targeting BMP7 in NRK52e. Int. J. Mol. Sci. 2015, 16, 27945-27955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126075
Liu Z, Zhou Y, Yuan Y, Nie F, Peng R, Li Q, Lyu Z, Mao Z, Huang L, Zhou L, et al. MiR542-3p Regulates the Epithelial-Mesenchymal Transition by Directly Targeting BMP7 in NRK52e. International Journal of Molecular Sciences. 2015; 16(11):27945-27955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126075
Chicago/Turabian StyleLiu, Zhicheng, Yuru Zhou, Yue Yuan, Fang Nie, Rui Peng, Qianyin Li, Zhongshi Lyu, Zhaomin Mao, Liyuan Huang, Li Zhou, and et al. 2015. "MiR542-3p Regulates the Epithelial-Mesenchymal Transition by Directly Targeting BMP7 in NRK52e" International Journal of Molecular Sciences 16, no. 11: 27945-27955. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126075