Randomized Controlled Trial of Darbepoetin α Versus Continuous Erythropoietin Receptor Activator Injected Subcutaneously Once Every Four Weeks in Patients with Chronic Kidney Disease at the Pre-Dialysis Stage
Abstract
:1. Introduction
2. Results and Discussion
| Patient Characteristic | DA Continuation Group (n = 10) | CERA Changeover Group (n = 10) | p Value |
|---|---|---|---|
| Age (years old) | 73.4 ± 8.2 | 69.4 ± 4.0 | 0.410 |
| Body weight (kg) | 51.7 ± 1.9 | 53.5 ± 3.0 | 0.623 |
| Systolic blood pressure (mmHg) | 134 ± 6 | 143 ± 8 | 0.330 |
| Diastolic blood pressure (mmHg) | 72 ± 5 | 73 ± 4 | 0.864 |
| Hemoglobin level (g/dL) | 11.2 ± 0.2 | 11.1 ± 0.1 | 0.615 |
| Reticulocytes (%) | 6.7 ± 1.2 | 8.3 ± 1.5 | 0.421 |
| White blood cells (/μL) | 5190 ± 397 | 6160 ± 709 | 0.253 |
| Platelets (×104/μL) | 30.0 ± 13.5 | 30.2 ± 11.8 | 0.995 |
| Transferrin saturation (%) | 33.7 ± 3.0 | 33.1 ± 3.7 | 0.910 |
| Ferritin concentration (ng/mL) | 171.8 ± 20.8 | 151.3 ± 16.8 | 0.452 |
| Albumin concentration (g/dL) | 3.9 ± 0.1 | 3.9 ± 0.1 | 0.727 |
| Blood urea nitrogen concentration (mg/dL) | 51.6 ± 7.2 | 36.8 ± 4.4 | 0.101 |
| Creatinine concentration (mg/dL) | 3.07 ± 0.38 | 2.73 ± 0.48 | 0.585 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 17.3 ± 2.5 | 21.8 ± 3.4 | 0.306 |
| Uric acid (mg/dL) | 7.0 ± 0.6 | 5.8 ± 0.4 | 0.124 |
| Urinary protein excretion rate (g/g Cr) | 1.3 ± 0.4 | 1.5 ± 0.5 | 0.690 |


| Week | DA Continuation Group | CERA Changeover Group | |
|---|---|---|---|
| DA Doses (μg) | DA Doses (μg) | CERA Doses (μg) | |
| Week −12 | 45.0 ± 3.4 | 41.0 ± 3.8 | - |
| Week −8 | 45.0 ± 3.4 | 41.0 ± 3.8 | - |
| Week −4 | 45.0 ± 3.4 | 41.0 ± 3.8 | - |
| Week 0 | 45.0 ± 3.4 | - | 45.0 ± 3.3 |
| Week 4 | 49.0 ± 3.7 | - | 52.5 ± 4.5 |
| Week 8 | 53.0 ± 4.7 | - | 50.0 ± 5.3 |
| Week 12 | 55.0 ± 5.4 | - | 45.0 ± 10.4 |
| Week 16 | 53.0 ± 5.6 | - | 47.5 ± 11.5 |
| Week 20 | 53.0 ± 5.6 | - | 47.5 ± 13.7 |
| Week 24 | 51.0 ± 5.7 | - | 50.0 ± 13.4 |
| Week 28 | 53.0 ± 5.6 | - | 52.5 ± 13.1 |
| Week 32 | 49.0 ± 8.5 | - | 52.5 ± 13.1 |
| Week 36 | 50.0 ± 10.0 | - | 50.0 ± 13.9 |
| Week 40 | 53.0 ± 9.4 | - | 47.5 ± 14.6 |
| Week 44 | 53.0 ± 9.4 | - | 52.5 ± 13.1 |
| Week 48 | 55.0 ± 8.9 | - | 52.5 ± 13.1 |
| Week | DA Continuation Group | CERA Changeover Group | ||
|---|---|---|---|---|
| Transferrin Saturation (%) | Ferritin Concentration (ng/mL) | Transferrin Saturation (%) | Ferritin Concentration (ng/mL) | |
| Week −12 | 36.3 ± 3.4 | 172.8 ± 20.6 | 36.7 ± 4.1 | 167.7 ± 24.5 |
| Week −8 | 43.8 ± 7.2 | 160.2 ± 20.3 | 32.2 ± 4.5 | 174.4 ± 29.6 |
| Week −4 | 35.4 ± 3.9 | 167.0 ± 19.0 | 35.3 ± 3.5 | 164.4 ± 25.7 |
| Week 0 | 33.7 ± 3.0 | 171.8 ± 20.8 | 33.1 ± 3.7 | 151.3 ± 16.8 |
| Week 4 | 34.4 ± 3.6 | 162.2 ± 12.3 | 36.4 ± 3.2 | 140.0 ± 16.1 |
| Week 8 | 33.5 ± 2.5 | 162.8 ± 16.8 | 32.5 ± 2.4 | 146.3 ± 15.7 |
| Week 12 | 33.2 ± 2.6 | 148.2 ± 17.1 | 38.6 ± 4.8 | 138.6 ± 17.6 |
| Week 16 | 32.4 ± 2.2 | 150.9 ± 17.5 | 33.8 ± 2.5 | 145.1 ± 16.3 |
| Week 20 | 33.7 ± 2.8 | 136.9 ± 15.1 | 40.4 ± 4.8 | 172.0 ± 28.8 |
| Week 24 | 32.7 ± 2.8 | 161.5 ± 18.6 | 40.7 ± 3.4 | 170.3 ± 24.6 |
| Week 28 | 32.1 ± 3.0 | 148.8 ± 11.7 | 39.5 ± 3.7 | 155.7 ± 20.0 |
| Week 32 | 36.6 ± 1.7 | 154.0 ± 10.0 | 37.6 ± 3.9 | 160.2 ± 18.5 |
| Week 36 | 35.2 ± 2.8 | 156.3 ± 16.3 | 35.6 ± 2.9 | 143.9 ± 14.5 |
| Week 40 | 35.1 ± 2.5 | 161.2 ± 21.5 | 29.8 ± 3.2 | 159.2 ± 25.4 |
| Week 44 | 28.4 ± 2.6 | 157.7 ± 19.6 | 35.2 ± 2.7 | 161.7 ± 26.0 |
| Week 48 | 34.0 ± 3.3 | 163.7 ± 21.2 | 35.6 ± 3.4 | 158.6 ± 16.4 |
3. Experimental Section
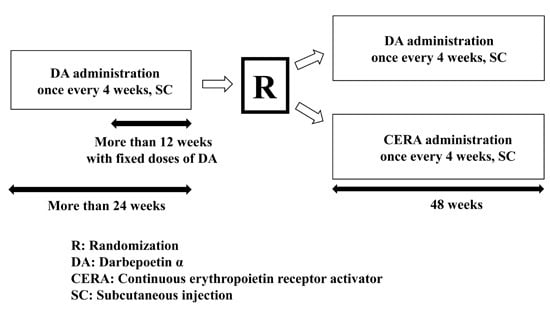
3.1. Study Design
| Dose of DA in the Four Weeks before the Study (μg) | Initial Dose (μg) | |
|---|---|---|
| DA Continuation Group | CERA Changeover Group | |
| 20 | 20 | 25 |
| 30 | 30 | 25 |
| 40 | 40 | 50 |
| 60 | 60 | 50 |

| Hemoglobin Level | Increase of Hemoglobin Level in Four Weeks | Increase of Hemoglobin Level in Four Weeks | Dosing Adjustment of Erythropoiesis-Stimulating Agent * |
|---|---|---|---|
| 13 g/dL or more | - | - | Interruption of treatment ** |
| 12.5 g/dL or more up to 13.0 g/dL | - | - | Dose reduction |
| Over 11.0 g/dL but under 12.5 g/dL | Under 1.0 g/dL | Under 0.5 mg/dL | Same dose |
| Over 0.5 mg/dL but under 1.0 mg/dL | Same dose | ||
| Over 1.0 mg/dL | Lower dose | ||
| Over 1.0 g/dL | Under 0.5 mg/dL | Same dose | |
| Over 0.5 mg/dL but under 1.0 mg/dL | Lower dose | ||
| Under 0.5 mg/dL | Lower dose | ||
| Under 11.0 g/dL | Under 1.0 g/dL | Under 0.5 mg/dL | Higher dose |
| Over 0.5 mg/dL but under 1.0 mg/dL | Higher dose | ||
| Over 1.0 mg/dL | Same dose | ||
| Over 1.0 g/dL | Under 0.5 mg/dL | Higher dose | |
| Over 0.5 mg/dL but under 1.0 mg/dL | Same dose | ||
| Under 0.5 mg/dL | Same dose |
3.2. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Kidney Foundation Kidney Disease Outcome Quality Initiative. Clinical practice guidelines for chronic kidney disease; evaluation, classification and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; de Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from kidney disease improving global outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: Summary of Recommendation Statements. Kidney Int. 2013, 84 (Suppl. S3), S5–S14. [Google Scholar]
- Takahashi, S.; Okada, K.; Yanai, M. The Kidney Early Evaluation Program (KEEP) of Japan: Results from the initial screening period. Kidney Int. 2010, 77, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Eckardt, K.U. Novel strategies for stimulating erythropoesis and potential new treatment for anemia. Lancet 2006, 368, 947–953. [Google Scholar] [CrossRef]
- Kleinman, K.S.; Schweitzer, S.U.; Perdue, S.T.; Bleifer, K.H.; Abels, R.I. The use of recombinant human erythropoietin in the correction of anemia in predialysis patients and its effect on renal function: A double-blind, placebo-controlled trial. Am. J. Kidney Dis. 1989, 14, 486–495. [Google Scholar] [CrossRef]
- Gouva, C.; Nikolopoulos, P.; Ioannidis, J.P.; Shimopoulos, K.C. Treating anemia early in renal failure patients slows the decline of renal function: A randomized controlled trial. Kidney Int. 2004, 66, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Tomonari, H.; Yoshida, H.; Hashimoto, T.; Kawanishi, Y.; Sakai, O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 2007, 77, 176–185. [Google Scholar] [CrossRef]
- Locatelli, F.; Reigner, B. CERA: Pharmacodynamics, pharmacokinesis and efficacy in patients with chronic kidney disease. Expert Opin. Investig. Drugs 2007, 16, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Walker, R.; Provenzano, R.; de Alvaro, F.; Locay, H.R.; Nader, P.C.; Locatelli, F.; Dougherty, F.C.; Beyer, U.; ARCTOS study investigators. C.E.R.A. Corrects anemia in patients with chronic kidney disease not on dialysis: Results of a randomized clinical trial. Clin. J. Am. Soc. Nephrol. 2008, 3, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Locatelli, F.; Woitas, W.; Laville, M.; Tobe, S.W.; Provenzano, R.; Golper, T.A.; Ruangkanchanasetr, P.; Lee, H.Y.; Wu, K.D.; et al. C.E.R.A. once every 4 week corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis. Nephrol. Dial. Transplant. 2011, 26, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Kolmakova, E.; Fung, M.; Malecki, R.; Vinhas, J.; Dellanna, F.; Thomas, M.; Manamley, N.; Ferenczi, S. Darbepoetin alfa once monthly corrects anaemia in patients with chronic kidney disease not on dialysis. Nephrology 2014, 19, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Zamboli, P.; Chiodini, P.; Mascia, S.; Vitiello, S.; Stanzione, G.; Bertino, V.; Conte, G.; de Nicola, L. Conversion of darbepoetin to low doses of CERA maintains hemoglobin levels in non-dialysis chronic kidney disease patients. Blood Purif. 2010, 30, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yang, C.W.; Kim, Y.H.; Joo, K.W.; Yoo, T.H.; Lee, K.W.; Lee, S.H.; Moon, J.Y.; Shin, S.K.; Huh, W.; et al. Effect of conversion from ESA with shorter half-life to CERA once monthly for maintaining Hb concentration in predialysis CKD patients. Kidney Blood Press. Res. 2013, 37, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Hasumi, S.; Mandai, S.; Tanaka, T.; Shikuma, S.; Akita, W.; Mori, Y.; Sasaki, S. Effects of three kinds of erythropoiesis-stimulating agents on renal anemia in Japanese non-dialysis chronic kidney disease patients. Clin. Exp. Nephrol. 2014, 18, 755–762. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, T.; Okada, K.; Abe, M.; Tei, R.; Oikawa, O.; Maruyama, N.; Maruyama, T. Randomized Controlled Trial of Darbepoetin α Versus Continuous Erythropoietin Receptor Activator Injected Subcutaneously Once Every Four Weeks in Patients with Chronic Kidney Disease at the Pre-Dialysis Stage. Int. J. Mol. Sci. 2015, 16, 30181-30189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226229
Furukawa T, Okada K, Abe M, Tei R, Oikawa O, Maruyama N, Maruyama T. Randomized Controlled Trial of Darbepoetin α Versus Continuous Erythropoietin Receptor Activator Injected Subcutaneously Once Every Four Weeks in Patients with Chronic Kidney Disease at the Pre-Dialysis Stage. International Journal of Molecular Sciences. 2015; 16(12):30181-30189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226229
Chicago/Turabian StyleFurukawa, Tetsuya, Kazuyoshi Okada, Masanori Abe, Ritsukou Tei, Osamu Oikawa, Noriaki Maruyama, and Takashi Maruyama. 2015. "Randomized Controlled Trial of Darbepoetin α Versus Continuous Erythropoietin Receptor Activator Injected Subcutaneously Once Every Four Weeks in Patients with Chronic Kidney Disease at the Pre-Dialysis Stage" International Journal of Molecular Sciences 16, no. 12: 30181-30189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226229