Effects of Heat Stress on Metabolite Accumulation and Composition, and Nutritional Properties of Durum Wheat Grain
Abstract
:1. Introduction
2. Results
2.1. Effects of Heat Stress on Yield-Related and Qualitative/Nutritional Traits
| Seeds | Trait | G | T | G × T | “Primadur” | “T1303” | ||
|---|---|---|---|---|---|---|---|---|
| Control | Heat Shocked | Control | Heat Shocked | |||||
| Mature | Protein content (%) | **** | *** | ** | 14.94 ± 0.52 c | 15.04 ± 0.25 c | 16.74 ± 0.42 b | 19.00 ± 0.33 a |
| Carotenoids (µg/g·dw) | *** | ns | ns | 9.26 ± 0.54 a | 8.67± 0.23 a | 3.5 ± 0.49 b | 3.56 ± 0.04 b | |
| Anthocyanins (µg/g·dw) | - | - | - | nd | nd | 9.3 ± 0.54 b | 16 ± 0.73 a | |
| Antioxidant activity (mM Trolox/kg·dw) | ns | *** | *** | 13.64 ± 0.02 b | 13.85 ± 0.13 b | 12.95 ± 0.13 b | 14.2 ± 0.28 a | |
| Individual grain weight (mg·dw) | **** | **** | ns | 28.2 ± 0.1 c | 25.4 ± 1 d | 52.2 ± 0.32 a | 47.8 ± 1.3 b | |
| Grain yield per spike (mg·dw) | *** | *** | ns | 1410 ± 38 a | 1184 ± 89 b | 1210 ± 13 b | 1093 ± 5.8 b | |
| Grain number per spike | **** | ns | ns | 50 ± 2 a | 47 ± 5 a | 23 ± 1 b | 23 ± 1 b | |
| Immature | Individual grain weight (mg·dw) | **** | ns | ns | 10.8 ± 0.97 b | 11.2 ± 0.81 b | 15.9 ± 0.41 a | 16.3 ± 0.24 a |
| Grain yield per spike (mg·dw) | **** | ns | ns | 513 ± 20.1 a | 536 ± 22.3 a | 354 ± 6.5 b | 370 ± 19.1 b | |
| Grain number per spike | **** | ns | ns | 49 ± 2 a | 48 ± 4 a | 23 ± 1 b | 22 ± 2 b | |
2.2. Analysis of Variance for Classes of Polar Metabolites
| Class of Metabolite | G | T | t | G × T | G × t | T × t | G × T × t |
|---|---|---|---|---|---|---|---|
| Amino acids | *** | *** | *** | *** | *** | *** | *** |
| N-compounds | *** | ** | *** | *** | *** | ns | ns |
| Organic acids | *** | ** | *** | *** | *** | * | *** |
| Sugars | *** | * | *** | *** | *** | ns | ** |
| Sugar alcohols | *** | * | *** | *** | *** | ns | ** |
2.3. Analysis of Variance for Individual Metabolites

| Metabolite | ANOVA Significance | |||||
|---|---|---|---|---|---|---|
| Immature Seeds | Mature Seeds | |||||
| G | T | G × T | G | T | G × T | |
| Amino acids | **** | *** | *** | ns | ns | **** |
| Valine | **** | ns | ns | * | **** | **** |
| Alanine | **** | **** | * | ns | ns | **** |
| Asparagine | **** | ** | *** | ** | ns | **** |
| Aspartic acids | **** | *** | **** | ns | ns | **** |
| Glutamic acid | **** | ns | ns | ns | ns | **** |
| Isoleucine | *** | ** | ns | ** | ns | **** |
| Serine | ** | ns | ns | ns | ns | **** |
| Glycine | **** | ** | ** | ns | ** | **** |
| Threonine | **** | ns | ns | ns | ns | **** |
| Leucine | ns | ns | ns | ns | ns | * |
| β-Alanine | **** | ns | ns | ns | ns | * |
| Phenylalanine | **** | **** | **** | ns | ns | **** |
| Tryptophan | **** | ** | ns | ns | ns | *** |
| Tyrosine | **** | ** | * | ns | ns | **** |
| GABA | **** | ** | ** | ns | ns | **** |
| N-compounds | *** | * | * | * | ** | **** |
| Putrescine | **** | ** | ** | * | * | * |
| Cadaverine | **** | *** | *** | * | *** | ** |
| Spermidine | **** | ns | ns | ns | ns | **** |
| Organic acids | **** | ** | *** | ns | ns | **** |
| Citric acid | **** | ns | *** | ns | ns | **** |
| Ferulic acid | **** | **** | **** | * | * | **** |
| Fumaric acid | **** | ** | **** | ** | * | **** |
| Malic acid | **** | * | **** | ** | ** | **** |
| Nicotinic acid | **** | ns | ns | ns | ns | *** |
| Oxalic acid | **** | ns | **** | ns | ns | *** |
| Quinic acid | **** | ns | ns | ns | ns | **** |
| Saccharic acid | **** | * | * | * | ** | **** |
| Shikimic acid | **** | ns | ns | * | ns | ns |
| 3PGA | **** | **** | **** | **** | **** | **** |
| Sugars | **** | ns | *** | * | * | **** |
| Raffinose | **** | ns | ** | ns | ns | **** |
| Sucrose | ns | *** | ** | ns | ns | **** |
| Glucose | **** | **** | **** | ** | ** | **** |
| Glucose 6-phosphate | **** | ns | ns | * | * | ** |
| Fructose | **** | *** | **** | ns | ns | **** |
| Fructose 6-phosphate | **** | ns | ns | ns | ns | *** |
| Ribose | **** | *** | *** | ns | ns | **** |
| Mannose | **** | ** | ** | ns | ns | **** |
| Palatinose and maltitol | **** | **** | **** | **** | **** | **** |
| Xylose and lyxose | **** | **** | **** | ns | ns | **** |
| Maltose and turanose | **** | *** | *** | ** | * | **** |
| Sugar alcohols | **** | ns | ns | ns | ** | **** |
| Mannitol | **** | * | ** | ** | **** | **** |
| myo-Inositol | **** | * | ** | ns | ns | **** |
| Glycerol | **** | ns | ns | *** | **** | **** |
2.4. Metabolite Changes in Response to Heat Stress in the Two Durum Wheat Genotypes
| Metabolite Class | Metabolite | Metabolite Content According to Seeds, Genotype and Heat Stress (µg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Immature Seeds | Mature Seeds | ||||||||
| “Primadur” | “T1303” | “Primadur” | ‘T1303’ | ||||||
| Control | Heat Shocked | Control | Heat Shocked | Control | Heat Shocked | Control | Heat Shocked | ||
| Amino acids | Valine | 270.70 ± 21.41 b | 267.00 ± 7.2 b | 472.61 ± 33.09 a | 417.28 ± 31.48 a | 4.61 ± 0.36 c | 35.71 ± 1.9 a | 18.64 ± 0.78 b | 12.41 ± 4.65 b |
| Alanine | 564.62 ± 141.78 c | 872.74 ± 163.77 b | 1101.31 ± 64.53 b | 1755.42 ± 33.55 a | 2.30 ± 1.51 b | 52.33 ± 10.07 a | 46.00 ± 13.9 a | 2.54 ± 0.64 a | |
| Asparagine | 1288.58 ± 240.59 b | 2937.24 ± 463.81 a | 762.11 ± 108.55 c | 399.26 ± 28.08 c | 94.80 ± 22.3 d | 785.64 ± 153.1 b | 1130.97 ± 198.7 a | 251.81 ± 17.4 c | |
| Aspartic acid | 905.30 ± 99.02 b | 1594.59 ± 91.14 a | 234.81 ± 36.14 c | 109.90 ± 13.69 c | 12.18 ± 6.4 b | 731.34 ± 128.6 a | 651.2 ± 134.8 a | 19.89 ± 2 b | |
| Glutamic acid | 1783.16 ± 38.36 b | 2044.83 ± 282.1 a | 225.47 ± 12.81 c | 170.25 ± 11.73 c | 48.8 ± 10.14 b | 922.88 ± 188.47 a | 843.14 ± 71.3 a | 58.70 ± 4.02 b | |
| Isoleucine | 102.73 ± 5.5 a | 79.90 ± 12.47 b | 145.72 ± 14.61 a | 122.15 ± 9.02 a | 1.82 ± 1.36 b | 7.56 ± 1.25 a | 8.91 ± 0.85 a | 5.53 ± 0.55 a | |
| Serine | 1288.70 ± 275.98 a | 1239.40 ± 200.18 a | 816.27 ± 40.28 b | 871.37 ± 50.38 b | 11.49 ± 0.37 b | 507.24 ± 111.58 a | 452.58 ± 102.7 a | 43.95 ± 35.5 b | |
| Glycine | 388.48 ± 30.45 b | 477.34 ± 4.29 a | 364.77 ± 15.54 c | 349.93± 12.33 c | 0.93 ± 0.04 c | 11.46 ± 1.9 a | 7.08 ± 1.45 b | 2.22 ± 1.53 c | |
| Threonine | 377.24 ± 26.29 a | 370.0 ± 18.08 a | 114.34 ± 12.16 b | 111.11 ± 4.75 b | 24.90 ± 1.46 b | 188.53 ± 36.85 a | 193.26 ± 9.17 a | 24.96 ± 18.12 b | |
| Leucine | 54.16 ± 12.83 | 44.22 ± 10.61 | 66.34 ± 9.04 | 57.12 ± 8.96 | 1.76 ± 0.51 | 3.87 ± 1.54 | 4.11 ± 0.8 | 2.73 ± 0.56 | |
| β-Alanine | 36.84 ± 0.58 a | 41.59 ± 4.86 a | 3.25 ± 0.3 b | 2.31 ± 0.06 b | 1.29 ± 1.8 b | 24.36 ± 4.9 a | 20.39 ± 1.8 a | 0.15 ± 0.04 b | |
| Phenyl-alanine | 122.70 ± 5.13 b | 184.59± 2.87 a | 17.56 ± 1.47 c | 15.45 ± 0.53 c | 27.73 ± 0.4 b | 74.41 ± 10.9 a | 75.69 ± 9 a | 33.67 ± 0.4 b | |
| Tryptophan | 374.54 ± 20.03 c | 495.99 ± 76.84 c | 885.13 ± 119.2 b | 1174.36 ± 76.73 a | 217.38 ± 15.8 b | 328.26 ± 14.5 a | 288.04 ± 51.8 a | 177.44 ± 32.7 c | |
| Tyrosine | 42.84 ± 2.79 b | 59.27 ± 7.69 a | 7.09 ± 1.32 c | 8.64 ± 1.16 c | 2.27 ± 1.2 b | 23.78 ± 7.4 a | 25.42 ± 4.8 a | 4.30 ± 2.8 b | |
| GABA | 787.32 ± 48.17 b | 1101.48 ± 114.79 a | 37.75 ± 6.45 c | 14.06 ± 4.3 c | 8.44 ± 0.99 b | 403.25 ± 102.8 a | 335.83 ± 37.7 a | 7.54 ± 0.84 b | |
| N-compounds | Putrescine | 22.93 ± 3.8 b | 39.68 ± 7.3 a | 2.35 ± 0.2 c | 2.46 ± 0.4 c | 0.21 ± 0.05 b | 11.22 ± 6.7 a | 0.72 ± 0.56 b | 0.73 ± 0.3 b |
| Cadaverine | 7.13 ± 0.8 b | 10.49 ± 0.7 a | 0.52 ± 0.07 c | 0.43 ± 0.03 c | 0.52 ± 0.07 b | 3.48 ± 0.6 a | 0.82 ± 0.7 b | 1.59 ± 0.06 b | |
| Spermidine | 38.22 ± 1.7 a | 47.45 ± 16.4 a | 1.34 ± 0.2 b | 1.88 ± 1 b | 0.21 ± 0.05 b | 10.68 ± 5.1 a | 14.57 ± 2.8 a | 0.21 ± 0.05 b | |
| Organic acids | Citric acid | 459.28 ± 64.1 b | 634.92 ± 41.6 a | 177.07 ± 12.5 c | 81.50 ± 16.1 d | 1.74 ± 0.18 b | 287.19 ± 94.4 a | 274.99 ± 28 a | 4.99 ± 0.42 b |
| Ferulic acid | 10.32 ± 0.7 b | 19.34 ± 0.3 a | 0.71 ± 0.08 c | 0.50 ± 0.15 c | 0.24 ± 0.3 c | 8.70 ± 1.5 a | 5.85 ± 1.1 b | 0.08 ± 0.01 c | |
| Fumaric acid | 177.35 ± 2.9 b | 217.43 ± 7.4 a | 97.94 ± 11.2 c | 18.34 ± 1.4 d | 27.47 ± 0.2 c | 153 ± 16 a | 113.33 ± 5.1 b | 23.34 ± 10.8 c | |
| Malic acid | 3644.35 ± 217.6 b | 4643.75 ± 122.3 a | 1423.95 ± 82.4 c | 998.79 ± 156.1 d | 388.73 ± 11.8 c | 1714.39 ± 371 b | 2485.56 ± 113.5 a | 384.11 ± 13.7 c | |
| Nicotinic acid | 12.5 ± 1.6 a | 13.61 ± 0.2 a | 7.70 ± 0.3 b | 7.05 ± 0.7 b | 3.70 ± 1.1 c | 5.97 ± 0.22 b,c | 7.23 ± 1.5 a,b | 3.50 ± 0.8 c,d | |
| Oxalic acid | 195.75 ± 4.2 c | 278.72 ± 15.9 a | 227.04 ± 6.7 b | 133.30 ± 10.7 d | 190.53 ± 58.6 b | 403.1 ± 44.6 a | 355.3 ± 56.7 a,c | 172.87 ± 60.9 b,c | |
| Quinic acid | 159.48 ± 52.6 a | 160.95 ± 20 a | 4.63 ± 0.24 b | 3.65 ± 0.05 b | 1.1 ± 0.61 b | 33.52 ± 14.1 a | 40.93 ± 10.1 a | 0.24 ± 0.01 b | |
| Saccharic acid | 6478.77 ± 828.1 b | 9090.44 ± 1296.2 a | 1573.27 ± 22.5 c | 1299.86 ± 71.3 c | 108.98 ± 8.9 c | 5359.32 ± 277.3 a | 4525.53 ± 335.5 b | 188.93 ± 5.7 c | |
| Shikimic acid | 22.21 ± 4.4 a | 26.66 ± 10.4 a | 1.46 ± 0.3 b | 0.97 ± 0.1 b | 8.86 ± 0.26 | 11.59 ± 2.7 | 15.36 ± 4.3 | 14.40 ± 1.8 | |
| 3PGA | 5.67 ± 0.2 a | 3.7 ± 0.1 b | 0.38 ± 0.07 c | 0.38 ± 0.04 c | 26.19 ± 0.7 b | 4.17 ± 0.6 c | 4.49 ± 1.5 c | 35.63 ± 0.8 a | |
| Sugars | Raffinose | 171 × 103 ± 23,837 a | 194.2 × 103 ± 17,216 a | 109.9 × 103 ± 12,833 b | 51.1 × 103 ± 3581 c | 23.6 × 103 ± 488 b | 79.3 × 103 ± 17,194 a | 60 × 103 ± 11,498 a | 29.1 × 103 ± 1801 b |
| Sucrose | 74.1 × 103 ± 4287 b | 93.6 × 103 ± 6549 a | 79.9 × 103 ± 1056 b | 83.7 × 103 ± 958 a,b | 22.8 × 103 ± 367 b | 54.7 × 103 ± 7948 a | 50.5 × 103 ± 4090 a | 23.4 × 103 ± 546 b | |
| Glucose | 6333.51 ± 183 b | 8777.81 ± 208 a | 609.21 ± 38 c | 453.68 ± 123 c | 5.26 ± 0.4 c | 5626.15 ± 517 a | 3179.70 ± 763 b | 8.00 ± 0.5 c | |
| Glucose 6-phosphate | 48.37 ± 9.3 a | 52.1 ± 11.1 a | 0.32 ± 0.04 b | 0.18 ± 0.07 b | 0.18 ± 0.07 c | 41.67 ± 12 b | 141.7 ± 71 a | 0.32 ± 0.03 c | |
| Fructose | 15,186.55 ± 1824 b | 22,969.97 ± 188 a | 1178,03 ± 395 c | 808.44 ± 100 c | 502.52 ± 376 b | 13,476.94 ± 2103 a | 8658.34 ± 3269 a | 284.88 ± 54 b | |
| Fructose 6-phosphate | 919.3 ± 139 a | 736.7 ± 307 a | 3.8 ± 0.8 b | 2.63 ± 0.5 b | 0.86 ± 0.56 b | 345.09 ± 135 a | 316.75 ± 105 a | 2.8 ± 0.71 b | |
| Ribose | 257.68 ± 28.6 b | 367.70 ± 17.2 a | 15.72 ± 1.2 c | 17.09 ± 0.4 c | 4.33 ± 0.2 b | 127.98 ± 15 a | 140.92 ± 32.5 a | 2.48 ± 0.08 b | |
| Mannose | 1749.64 ± 223 b | 2780.72 ± 294 a | 124.04 ± 37 c | 117.94 ± 11 c | 27.44 ± 1.3 b | 1596.85 ± 34.3 a | 1461.67 ± 284 a | 31.77 ± 8.8 b | |
| Palatinose and maltitol | 23.19 ± 2.5 b | 74.29 ± 8.7 a | 9.53 ± 2.1 c | 15.14 ± 1.9 c | 20.30 ± 9.5 c | 22.91 ± 9.5 b,c | 134.31 ± 17 a,b | 21.15 ± 5.4 c,d | |
| Xylose and lyxose | 236.62 ± 17.8 b | 914.38 ± 51 a | 42.55 ± 2.8 c | 35.72 ± 3 c | 6.80 ± 0.6 c | 229.21 ± 38.6 a | 155.01 ± 37.5 b | 17.26 ± 2.4 c | |
| Maltose and turanose | 32.6 × 103 ± 2528 b | 50 × 103 ± 4071 a | 117.6 × 103 ± 348 c | 20.1 × 103 ± 3258 c | 7176.69 ± 132 c | 30.1 × 103 ± 4058 a | 21.3 × 103 ± 1875 b | 4382.71 ± 399 d | |
| Sugar alcohols | Mannitol | 133.43 ± 12.33 a | 140.58 ± 6 a | 41.39 ± 3.7 b | 11.96 ± 1.1 c | 2.90 ± 0.5 d | 87.08 ± 5.5 a | 62.05 ± 3.3 b | 10.37 ± 2.1 c |
| myo-Inositol | 335.32 ± 12.9 b | 364.13 ± 6.5 a | 61.86 ± 3 c | 52.99 ± 1.3 c | 10.96 ± 0.88 b | 256.76 ± 48.8 a | 227.33 ± 43.1 a | 14.98 ± 0.45 b | |
| Glycerol | 1698.32 ± 73 a | 1733.06 ± 128 a | 1181.24 ± 60 b | 1229.17 ± 79 b | 16.06 ± 0.96 c | 128.94 ± 14.9 a | 40.61 ± 4.6 b | 49.77 ± 2.9 b | |
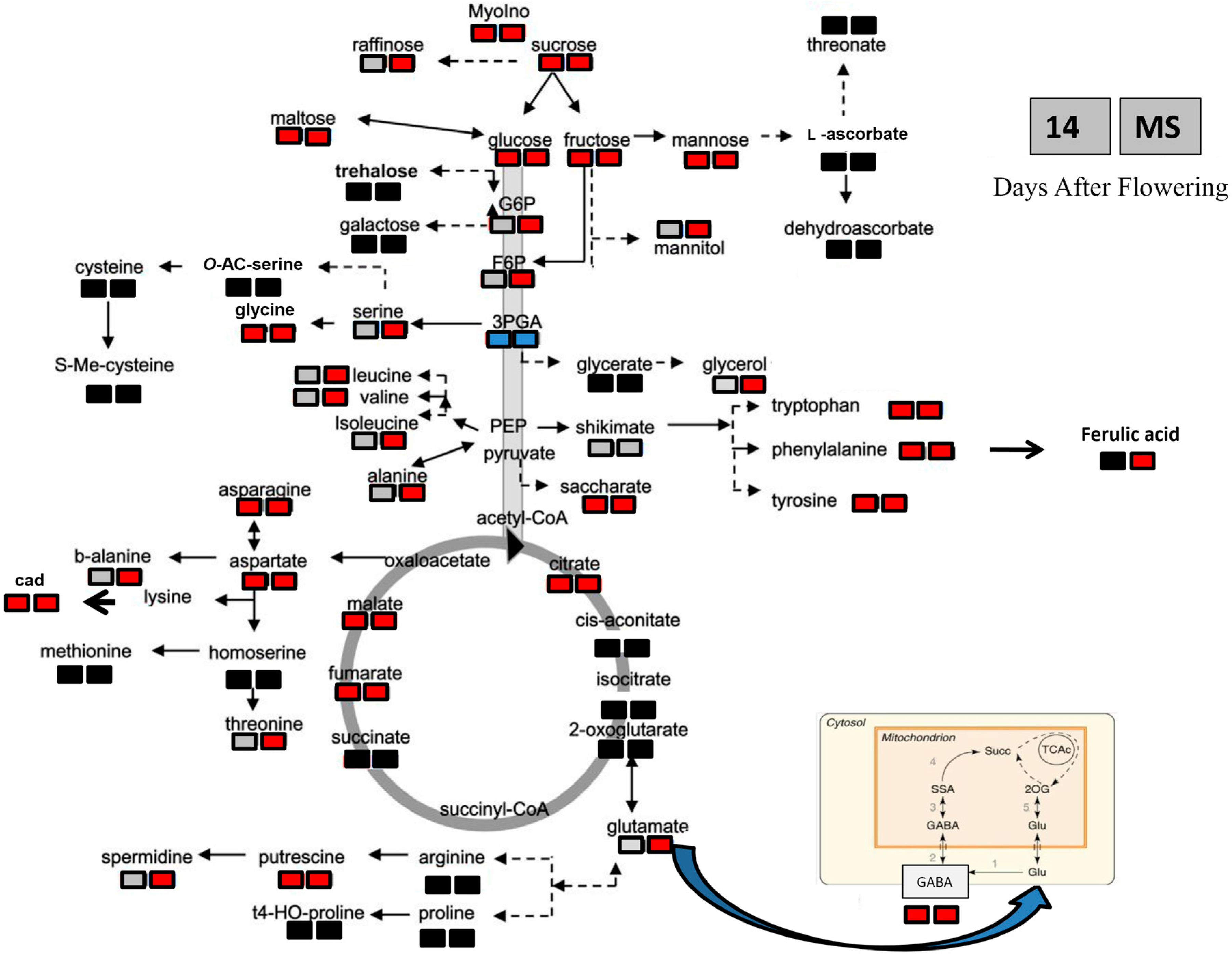
3. Discussion
3.1. Heat Shock Applied Early during Seed Development Produces Different Long-Term Effects in Two Durum Wheat Genotypes
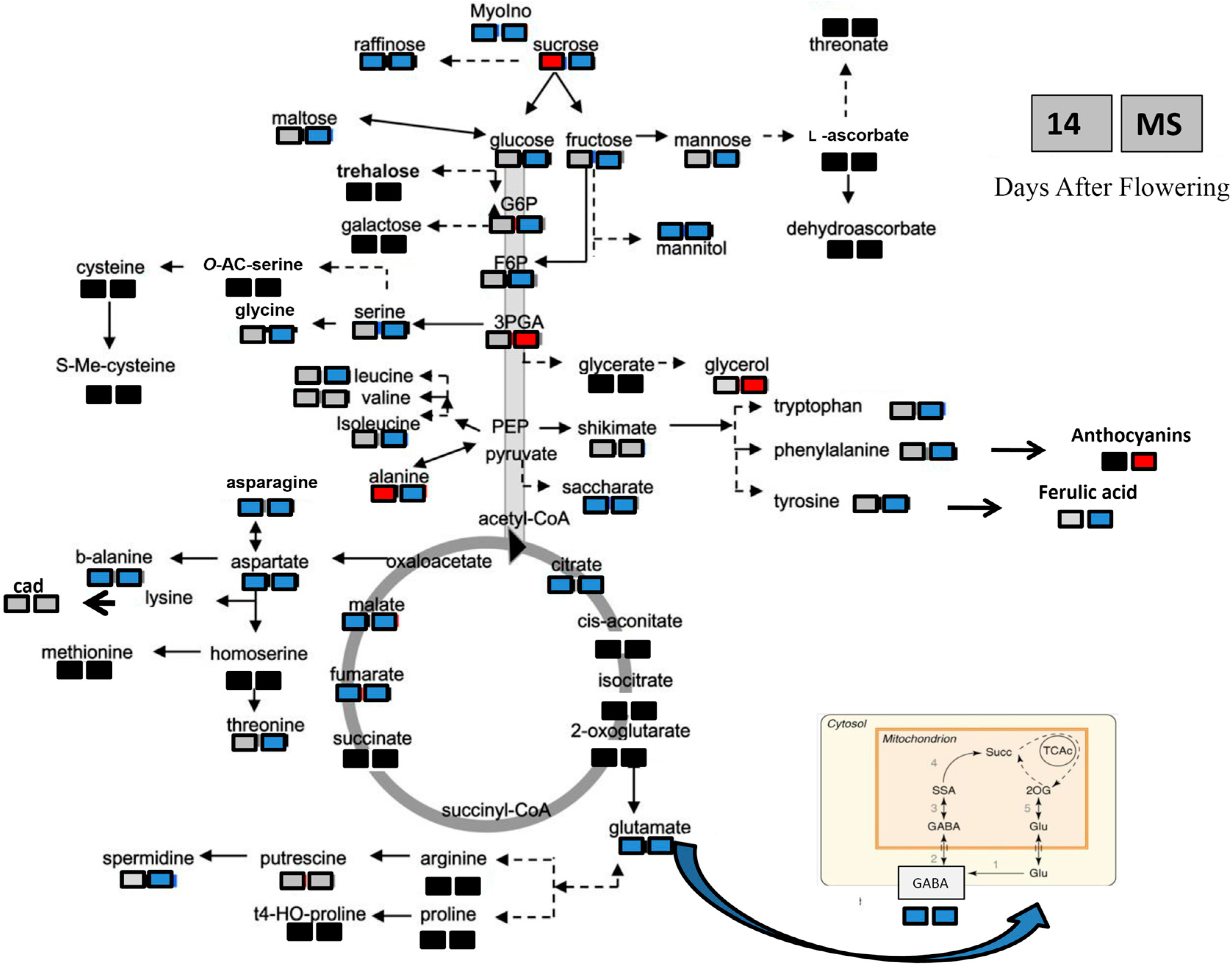
3.2. Effects of Heat Stress on Antioxidant Activity and Nutritional Properties of Durum Wheat Grain
4. Materials and Methods
4.1. Plant Material and Heat-Stress Treatment
4.2. Yield-Related and Qualitative/Nutritional Traits
4.2.1. Yield-Related Traits
4.2.2. Protein Content
4.2.3. Total Anthocyanin Content Using the pH Differential Method
4.2.4. Total Carotenoid Content
4.2.5. In-Vitro Determination of Antioxidant Activity
4.3. Analysis of Polar Metabolites
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li, Z.; Békés, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech. J. Food Sci. 2011, 29, 117–128. [Google Scholar]
- Hansen, J.; Sato, M.; Ruedy, R. Perception of climate change. Proc. Natl. Acad. Sci. USA 2012, 109, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.V.; Zhang, X.; Peterson, T.C.; Caesar, J.; Gleason, B.; Tank, A.; Haylock, M.; Collins, D.; Trewin, B.; Rahimzadeh, F.; et al. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 2006, 111, 1–22. [Google Scholar] [CrossRef]
- Hennessy, K.; Fawcett, R.; Kirono, D.; Mpelasoka, F.; Jones, D.; Bathols, J.; Whetton, P.; Stafford Smith, M.; Howden, M.; Mitchell, C.; et al. An Assessment of the Impact of Climate Change on the Nature and Frequency of Exceptional Climatic Events. Available online: http://www.worldcat.org/title/assessment-of-the-impact-of-climate-change-on-the-nature-and-frequency-of-exceptional-climatic-events/oclc/277155278?referer=br&ht=edition (accessed on 16 December 2015).
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 1–17. [Google Scholar] [CrossRef]
- Wollenweber, B.; Porter, J.R.; Schellberg, J. Lack of interaction between extreme high-temperature events at vegetative and reproductive growth stages in wheat. J. Agron. Crop Sci. 2003, 189, 142–150. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C. Bread and durum wheat tolerance under heat stress: A synoptical overview. J. Food Agric. 2010, 22, 412–436. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Havlickova, L.; Panna, R.; Marè, C.; Borrelli, G.M.; Marone, D.; Perrotta, C.; Rampino, P.; de Bellis, L.; Curn, V.; et al. Different stress responsive strategies to drought and heat in two durum wheat cultivars with contrasting water use efficiency. BMC Genom. 2013, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Laino, P.; Shelton, D.; Finnie, C.; De Leonardis, A.M.; Mastrangelo, A.M.; Svensson, B.; Lafiandra, D.; Masci, S. Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics 2010, 10, 2359–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [PubMed]
- Asthir, B.; Bala, S.; Bains, N.S. Metabolic profiling of grain carbon and nitrogen in wheat as influenced by high temperature. Cereal Res. Commun. 2013, 41, 230–242. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Ficco, D.B.M.; Giuzio, L.; Pompa, M.; Cattivelli, L.; Flagella, Z. Durum wheat salt tolerance in relation to physiological, yield and quality characters. Cereal Res. Commun. 2011, 39, 525–534. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hort. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Yeum, K.J.; Russell, R.M. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.M.; De Leonardis, A.M.; Platani, C.; Troccoli, A. Distribution along durum wheat kernel of the components involved in semolina colour. J. Cereal Sci. 2008, 48, 494–502. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.S.; Young, J.C.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Digesù, A.M.; Platani, C.; Cattivelli, L.; Mangini, G.; Blanco, A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009, 50, 210–218. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Dietary lutein & zeaxanthin: Reducing the risk for macular degeneration. Agron. Food Ind. Hi-Tech. 2004, 15, 22–25. [Google Scholar]
- Singh, P.; Goyal, G.K. Dietary lycopene: Its properties and anticarcinogenic effects. Compr. Rev. Food Sci. Food Saf. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Yoshiko, S. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, F.; Ferrari, B.; Gianninetti, A. A study of biodiversity of flavonoid content in the rice caryopsis evidencing simultaneous accumulation of anthocyanins and proanthocyanidins in a black-grained genotype. J. Cereal Sci. 2010, 51, 28–34. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physiochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Sanchez, G.S.; Hernandez, D.R.; Lobato, N.R. Characterization of anthocyanin extracts from maize kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Insfran, D.; Brenes, C.H.; Serna Saldivar, S.O.; Talcott, S.T. Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products. Food Res. Int. 2006, 39, 696–703. [Google Scholar] [CrossRef]
- Žofajová, A.; Pšenáková, I.; Havrlentová, M.; Piliarová, M. Accumulation of total anthocyanins in wheat grain. Agricolture 2012, 58, 50–56. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; de Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; Finocchiaro, F.; Papa, R.; de Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailon, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Lorenc-Kukula, K.; Jafra, S.; Oszmianski, J.; Szopa, J. Ectopic expression of anthocyanin 5-O-glucosyltransferase in potato tuber causes increased resistance to bacteria. J. Agric. Food Chem. 2005, 53, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Abou-Arab, A.A.; Gamel, T.H.; Hucl, P.; Young, J.C.; Rabalski, I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J. Agric. Food Chem. 2008, 56, 11171–11177. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, H.; Zhang, D.; Li, P.; Ma, F. The role of anthocyanin in photoprotection and its relationship with the xanthophyll cycle and the antioxidant system in apple peel depends on the light conditions. Physiol. Plant 2013, 49, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Galvão, A.M.; Di Paolo, R.E.; Freitas, A.A.; Lima, J.C.; Quina, F.H.; Maçanita, A.L. Photochemistry of the hemiketal form of anthocyanins and its potential role in plant protection from UV-B radiation. Tetrahedron 2015, 71, 3157–3162. [Google Scholar] [CrossRef]
- Shipp, J.; Abdel-Aal, E.-S. Food applications and physiological effects of anthocyanins as functional food ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- De Pascual-Teresa, S.; Moreno, D.A.; Darcia-Viguera, C. Flavanols and anthocyanins in cardiovascular health. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed Jaafar, S.N.; Baron, J.; Siebenhandl-Ehn, S.; Rosenau, T.; Böhmdorfer, S.; Grausgruber, H. Increased anthocyanin content in purple pericarp × blue aleurone wheat crosses. Plant Breed. 2013, 132, 546–552. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Zeven, A.C. Wheats with purple and blue grains: A review. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Eticha, F.; Grausgruber, H.; Siebenhandl-Ehn, S.; Berghofer, E. Some agronomic and chemical traits of blue aleurone and purple pericarp wheat (Triticum L.). J. Agric. Sci. Technol. 2011, 1, 48–58. [Google Scholar]
- Sobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential and anti-oxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, hearth desease and cancer. Pharmacol. Rev. 2000, 52, 673–839. [Google Scholar] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ibtissem, B.; Abdelly, C.; Sfar, S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Lagishetty, C.V.; Naik, S.R. Polyamines: Potential anti-inflammatory agents and their possible mechanism of action. Ind. J. Pharmacol. 2008, 40, 121–125. [Google Scholar]
- Binh, P.N.T.; Soda, K.; Kawakami, M. Mediterranean diet and polyamine intake: Possible contribution of increased polyamine intake to inhibition of age-associated disease. Nutr. Diet. Suppl. 2011, 3, 1–7. [Google Scholar]
- Kalac, P.; Krausovà, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Larquè, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamine in foods: Development of food database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar]
- Dandrifosse, G.; Peulen, O.; el Khefif, N.; Deloyer, P.; Dandrifosse, A.C.; Grandfils, C. Are milk polyamines preventive agents against food allergy? Proc. Nutr. Soc. 2000, 59, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Knocz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Vigeolas, H.; Chinoy, C.; Zuther, E.; Blessington, B.; Geigenberger, P.; Domoney, C. Combined metabolomic and genetic approaches reveal a link between the polyamine pathway and albumin 2 in developing pea seeds. Plant Phys. 2008, 146, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Röhlig, R.M.; Eder, J.; Engel, K.H. Metabolite profiling of maize grain: Differentiation due to genetics and environment. Metabolomics 2009, 5, 459–477. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahshi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef]
- Alcázar, R.; Bitrián, M.; Zarza, X.; Tiburcio, A.F. Polyamine metabolism and signaling in plant abiotic stress protection. In Recent Advances in Pharmaceutical Sciences II, 2nd ed.; Chapter 3; Muñoz-Torrero, D., Haro, D., Vallès, J., Eds.; Transworld Research Network: Kerala, India, 2012; pp. 29–47. [Google Scholar]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Planas, J.; Saxena, T.; Zarza, X.; Bortolotti, C.; Cuevas, J.; Bitrián, M.; Tiburcio, A.F.; Altabella, T. Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol. Biochem. 2010, 48, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Ben Salem-Fnayou, A.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelekis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Phys. 2010, 167, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V-I.V.; Radyukina, N.L.; Shevyakova, N.I. Polyamines and stress: Biological role, metabolism and regulation. Russ. J. Plant Phys. 2003, 33, 583–604. [Google Scholar]
- Galiba, G.; Kocsy, G.; Kaur-Sawhney, R.; Sutka, J.; Galston, A.W. Chromosomal localisation of osmotic and salt stress induced differential alteration in polyamine content in wheat. Plant Sci. 1993, 92, 203–211. [Google Scholar] [CrossRef]
- Galiba, G.; Simon-Sarkadi, L.; Salgò, A.; Kocsy, G. Genotype-dependent adaptation of wheat varieties to water stress in vitro. J. Plant Physiol. 1989, 134, 730–735. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. γ Aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Phys. 2010, 51, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Breitkreuz, K.; Shelp, B.; Fischer, W.N.; Schwacke, R.; Rentsch, D. Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett. 1999, 280–284. [Google Scholar] [CrossRef]
- Choia, Y.; Jeonga, H.; Lee, J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007, 103, 130–138. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba Berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. Assoc. Off. Anal. Chem. Intern. 2004, 87, 129–145. [Google Scholar]
- Smith, P.T.; Kuo, T.M.; Crawford, C.G. Purification and characterization of galactinol synthase from mature zucchini squash leaves. Plant Physiol. 1991, 96, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hitz, W.D.; Carlson, T.J.; Kerr, P.S.; Sebastian, S.A. Biochemical and molecular characterization of a mutation that confers a decreased raffinosaccharide and phytic acid phenotype on soybean seeds. Plant Physiol. 2002, 128, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa-Yoloi, A.; Yabuta, Y.; Shigeoka, S. The contribution of carbohydrates including raffinose family oligosaccharides and sugar alcohols to protection of plant cells from oxidative damage. Plant Signal. Behav. 2008, 3, 1016–1018. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Einhorn Todd, C.; Cheng, L. Comparison of phenolic metabolism and primary metabolism between green “Anjou” pear and its bud mutation, red “Anjou”. Phys. Plant. 2014, 150, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Singh, R.K.; Navarre, D.A. Transcription factors, sucrose and sucrose matabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 2013, 64, 5115–5131. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Reichardt, B.; Shu, Q.; Engel, K.H. Metabolite profiling of colored rice (Oryza sativa L.) grains. J. Cereal Sci. 2012, 55, 112–119. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, H.Y.; Koh, H.J.; Ryu, S.N. Varietal difference of chemical composition in pigmented rice varieties. Korean J. Crop Sci. 2006, 51, 113–118. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Sciller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qui, Y.; Beta, T. Comparison of antioxidant activities of different coloured wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P.; Hatcher, D.W. Ultra-performance liquid chromatography (UPLC) quantification of carotenoids in durum wheat: Influence of genotype and environment in relation to the colour of yellow alkaline noodles (YAN). Food Chem. 2011, 125, 1510–1516. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H. Total Phenolic, Carotenoid, and Anthocyanin Content and Antioxidant Activity of Peach and Plum Genotypes. In Proceedings of the 6 International Peach Symposium, Santiago, Chile, 9–14 January 2005; Infante, R., Ed.; Acta Hort: Santiago, Chile, 2005; Volume 713, pp. 453–455. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.S.; Pandey, A.; Negi, R.; Pande, D.; Karki, K.; Khanna, H.D.; Khanna, S. Characterization and evaluation of antioxidant activity. Indian J. Res. 2011, 5, 40–47. [Google Scholar]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. In vitro antioxidant activity of dietary polyamines. Food Res. Intern. 2013, 51, 141–147. [Google Scholar] [CrossRef]
- Ichikawa, H.; Ichiyanagi, T.; Xu, B.; Yoshii, Y.; Nakajima, M.; Konisi, T. Antioxidant activity of anthocyanin extract from purple black rice. J. Med. Food 2001, 4, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’Asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Polyamine intake, dietary pattern, and cardiovascular disease. Med. Hypotheses 2010, 75, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sarkadi, L.; Kocsy, G.; Varhegyi, A.; Galiba, G.; de Ronde, J.A. Effect of drought stress at supraoptimal temperature on polyamine concentrations in transgenic soybean with increased proline levels. Z. Naturf. C 2006, 61, 833–839. [Google Scholar]
- Valluru, R.; van den Ende, W. Myo-inositol and beyond—Emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zhawar, V.K.; Kaur, N.; Gupta, A.K. Phytic acid and raffinose series oligosaccharides metabolism in developing chickpea seeds. Physiol. Mol. Biol. Plants 2011, 17, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Biancolatte, E.; Mariani, B.M. La rete nazionale di prove di confronto varietale tra frumenti duri. L'Informatore Agrario 1992, 48, 5–8. (In Italian) [Google Scholar]
- De Simone, V.; Menzo, V.; De Leonardis, A.M.; Ficco, D.B.M.; Trono, D.; Cattivelli, L.; De Vita, P. Different mechanisms control lipoxygenase activity in durum wheat kernels. J. Cereal Sci. 2010, 52, 121–128. [Google Scholar] [CrossRef]
- AACC International (American Association Cereal Chemists). Approved Methods of the AACC, 11th ed. Method 46-13.01. Available online: http://methods.aaccnet.org/summaries/46-13-01.aspx (accessed on 18 December 2015).
- AACC International (American Association Cereal Chemists). Approved Methods of the AACC, 11th ed. Method 14-50. Available online: http://methods.aaccnet.org/summaries/14-50-01.aspx.aspx (accessed on 18 December 2015).
- Beleggia, R.; Platani, C.; Nigro, F.; Cattivelli, L. A micro-method for the determination of yellow pigment content in durum wheat. J. Cereal Sci. 2010, 52, 106–110. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Beleggia, R.; Platani, C.; Nigro, F.; De Vita, P.; Cattivelli, L.; Papa, R. Effect of genotype, environment and genotype-by-environment interaction on metabolite profiling in durum wheat (Triticum durum Desf.) grain. J. Cereal Sci. 2013, 57, 183–192. [Google Scholar] [CrossRef]
- Braun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. In Climate Change and Crop Production, in MP Reynolds, 2nd ed.; CABI: Oxfordshire, UK, 2010; pp. 115–138. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; De Vita, P.; Mastrangelo, A.M. Effects of Heat Stress on Metabolite Accumulation and Composition, and Nutritional Properties of Durum Wheat Grain. Int. J. Mol. Sci. 2015, 16, 30382-30404. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226241
De Leonardis AM, Fragasso M, Beleggia R, Ficco DBM, De Vita P, Mastrangelo AM. Effects of Heat Stress on Metabolite Accumulation and Composition, and Nutritional Properties of Durum Wheat Grain. International Journal of Molecular Sciences. 2015; 16(12):30382-30404. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226241
Chicago/Turabian StyleDe Leonardis, Anna Maria, Mariagiovanna Fragasso, Romina Beleggia, Donatella Bianca Maria Ficco, Pasquale De Vita, and Anna Maria Mastrangelo. 2015. "Effects of Heat Stress on Metabolite Accumulation and Composition, and Nutritional Properties of Durum Wheat Grain" International Journal of Molecular Sciences 16, no. 12: 30382-30404. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161226241






