1. Introduction
The autosomal
DAZL gene is an important member of the Deleted in Azoospermia (DAZ) family (including
DAZ,
DAZL and
BOULE), which encode RNA-binding proteins of the RNA recognition motif (RRM) type and are involved in translation of polysomal mRNA [
1].
DAZL, which contains the conserved RRM repeat motif structural domain DAZ, was first discovered in the
Drosophila testis [
2]. Mouse
Dazl expression starts during the early stage of germ cell development, persists into meiosis, and is expressed both in the adult testis and ovaries [
3,
4]. The RNA-binding protein encoded by
Dazl is required for the completion of spermatocyte meiosis in the mouse [
5]. Houston
et al. [
6] found that
Xenopus DAZL was critically involved in primordial germ cell (PGC) development. Vogel
et al. [
7] determined that
Xenopus DAZL could only substitute for the murine homologue with respect to the early functions in the establishment of the germ cells. Reynolds
et al. [
8] found that in
Dazl knockout mice, meiotic prophase was arrested at the zygotene stage. Together these results indicate that
DAZL has a conserved role in germ cell development, regulation of germ cell differentiation, and is one of the important regulatory factors in the process of gamete formation.
Currently, many research groups are focused on germ cell gene regulation and inductive differentiation of PGCs from embryonic stem cells (ESCs)
in vitro. Yu [
9] induced mouse ESCs to differentiate into sperm-like cells and oocyte-like cells by overexpression of
Dazl. Haston [
10] had shown that
Dazl plays an important role in the process of differentiation from to germ cell
in vitro. Using overexpression of
Dazl Kee
et al. [
11] found that PGC formation could be promoted. Thus far, there have been a number of reports on mouse, human, and
Drosophila DAZL and identification of a large number of inducers of its expression; however, the reports on the chicken ortholog are not comprehensive enough. Our team has been committed for decades to studying the inductive differentiation of chicken male germ cells (MGCs)
in vitro. Unfortunately, the induction efficiency is low. To improve the induction efficiency, selecting the optimum inducers for chicken
DAZL gene expression is crucial.
Chicken, as a classic model anima of developmental biology, displays a unique process of embryonic development. As an experimental model system, chickens provide sufficient material for the study of embryonic germ cells and have more permissive rules than mammalian models. The 5' flanking region of the DAZL gene promoter was cloned upstream of a fluorescent reporter and transfected into DF-1 cells. The Dual-Luciferase® Reporter Assay System allowed quantification of the activity of the chicken DAZL promoter fragments and identification of the gene’s core promoter. The level of DAZL gene promoter activity was detected under different inducer treatments. Candidate optimal inducers were screened for their functional capacity in vitro to induce chicken ESCs to differentiate into MGCs, to provide the optimum inducer screening of the DAZL gene, and to lay the foundation for in vitro screening for inducers of ESC to MGC differentiation in the chicken.
3. Experimental Section
3.1. Materials and Reagents
Procedures involving animals and their care conformed to the U.S. National Institute of Health guidelines (NIH Pub. No. 85-23, revised 1996) and were approved by the laboratory-animal management and experimental-animal ethics committee of Yanzhou University.
Fertilized eggs of Suqin yellow chickens (
Gallus gallus domesticus) were purchased from the Chinese Academy of Agricultural Sciences Experimental Poultry Farm (Yangzhou, China). ESCs were collected from stage X embryos as described previously [
12].
The pEGFP-N1 vector is maintained by our laboratory. E. coli DH5α competent cells, gel extraction kits, and miniprep kits were purchased from Tiangen Biotech (Beijing, China). DL5000 DNA Marker Prime, STAR Max DNA Polymerase, T4 DNA ligase, and restriction endonucleases were purchased from Takara (Shiga, Japan). Expression vectors pGL3.0-Basic, pRL-SV40, and the dual-luciferase assay kit, Dual-Luciferase Reporter Assay System, were purchased from Promega Corporation (Madison, WI, USA). LipofectamineTM2000 was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Retinoic acid (RA), all-trans retinoic acid (ATRA), tamibarotene (Am80), estrogen (E2), and testosterone were purchased from SIGMA-Aldrich (St. Louis, MO, USA). Mouse bone morphogenetic protein 4 (BMP4) and follicle stimulating hormone (FSH) were purchased from ProSpec-Tany TechnoGene Ltd. (Rehovot, Israel). The mouse spermatogonial cell line (GC-1) and the chicken embryo fibroblast cell line (DF-1) were purchased from ATCC (Manassas, VA, USA). The remaining reagents were imported or domestic analytical grade. Primer synthesis and sequencing were conducted by Invitrogen.
3.3. Genomic Clone
Four fragments of the chicken DAZL gene promoter were amplified by polymerase chain reaction (PCR), forward primers (positions relative to the transcription start site, corresponding to the genomic positions chr2:34953772-34954681 for the longest amplicon) were: pEGFP-P0 (−932 to −39 bp): 5'-CGCATTAATTATGCACTCCAGTTGATCAGTTTAA-3', pGL-P1 (−932 to −39 bp): 5'-GGGGTACCTATGCACTCCAGTTGATCAGTTTAA-3', pGL-P2 (−647 to −39 bp): 5'-GGGTACCCCAGCCTGCTCCAGAGTATCCA-3', pGL-P3 (−383 to −39 bp): 5'-GGGGTACCCACACATCGCCCTTCGTCTT-3', pGL-P4 (−186 to −39 bp): 5'-GGGGTACCAAGAACTGCCTCTTTCGCAC-3', and common reverse primer: 5'-CCCTCGAGCAAACGAGGCCTTCAAGACAA-3'.
The PCR consisted of 35 cycles of gene amplification as follows: 98 °C for 10 s followed by 35 cycles composed of a denaturation step at 95 °C for 30 s, 56–62 °C for 30 s, and an elongation step at 72 °C for 40 s. The PCR products were resolved by electrophoresis on 1% agarose gels, and the appropriate band was excised and purified for the subsequent steps of cloning or sequence determination. Sequence analysis was performed with an automatic sequencer (ABI 377, Applied Biosystems/PE/Life Technologies, Grand Island, NY, USA).
3.4. Vector Construction
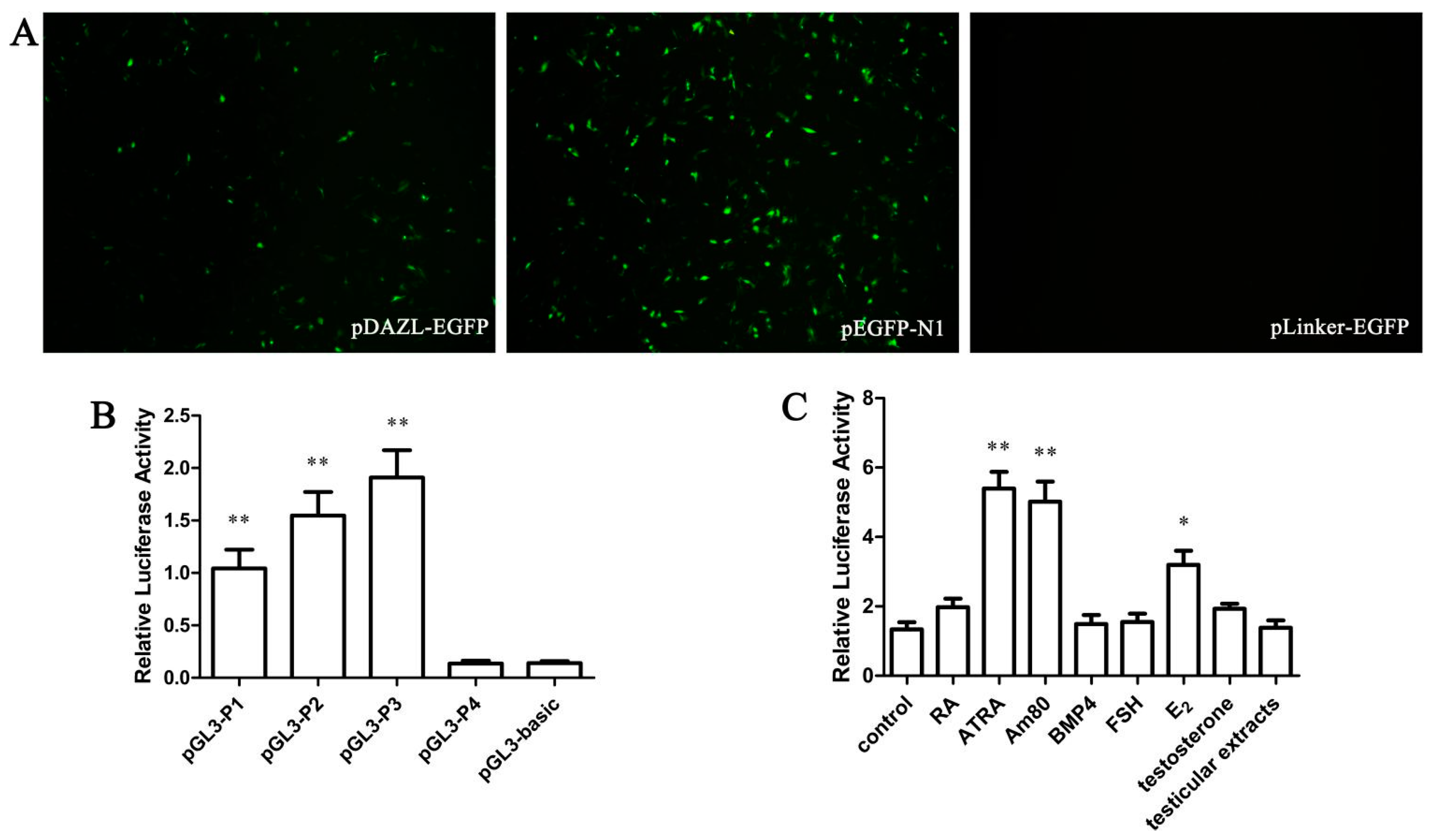
The DAZL gene 5' flanking area −932 to −39 bp was inserted into the pEGFP-N1 vector between AseI and XhoI restriction enzyme cutting sites and named pDAZL-EGFP. A negative control carrier was also built as pLinker-EGFP. Four fragments of the DAZL gene promoter region cloned previously were inserted into the dual-luciferase reporter gene carrier pGL3-Basic. All vectors were verified by AseI and XhoI restriction enzyme digestion and sequence analysis.
3.6. Cell Transfection
DF-1/GC-1 cells were inoculated into 24 wells, approximately 2.5 × 105 cells per well. According to the manufacturer’s instructions for LipofectamineTM2000, all recombinant plasmids and a reference plasmid pRL-SV40 (35:1) were transfected into DF-1/GC-1 cells. DF-1/GC-1 cells transfected with pGL3-basic and pRL-SV40 were selected as a negative group. The results from each group represent three replications. After 48 h, the Dual-Luciferase® Reporter Assay was performed.
After 6 h of transfection, cells were treated with RA (10−5 mol/L), ATRA (10−5 mol/L), Am80 (10−6 mol/L), BMP4 (40 ng/mL), FSH (0.1 IU/mL), E2 (1 μg/mL), testosterone (15 ng/mL), or testicular extract. The negative group was treated with the same volume of phosphate-buffered saline. After treatment for 48 h, cells were collected. The results of each group represent three replications.
3.7. Dual-Luciferase Activity Detection
Cells were collected in 70 μL PBS and the suspension was transferred to a 96-well plate. A Dual-Luciferase® Reporter Assay was performed to detect the activity of the DAZL gene promoter constructs. The relative luciferase activity representing the promoter activity is reported as the ratio of firefly luciferase value/renilla luciferase.
3.8. In Vitro Induction Experiment
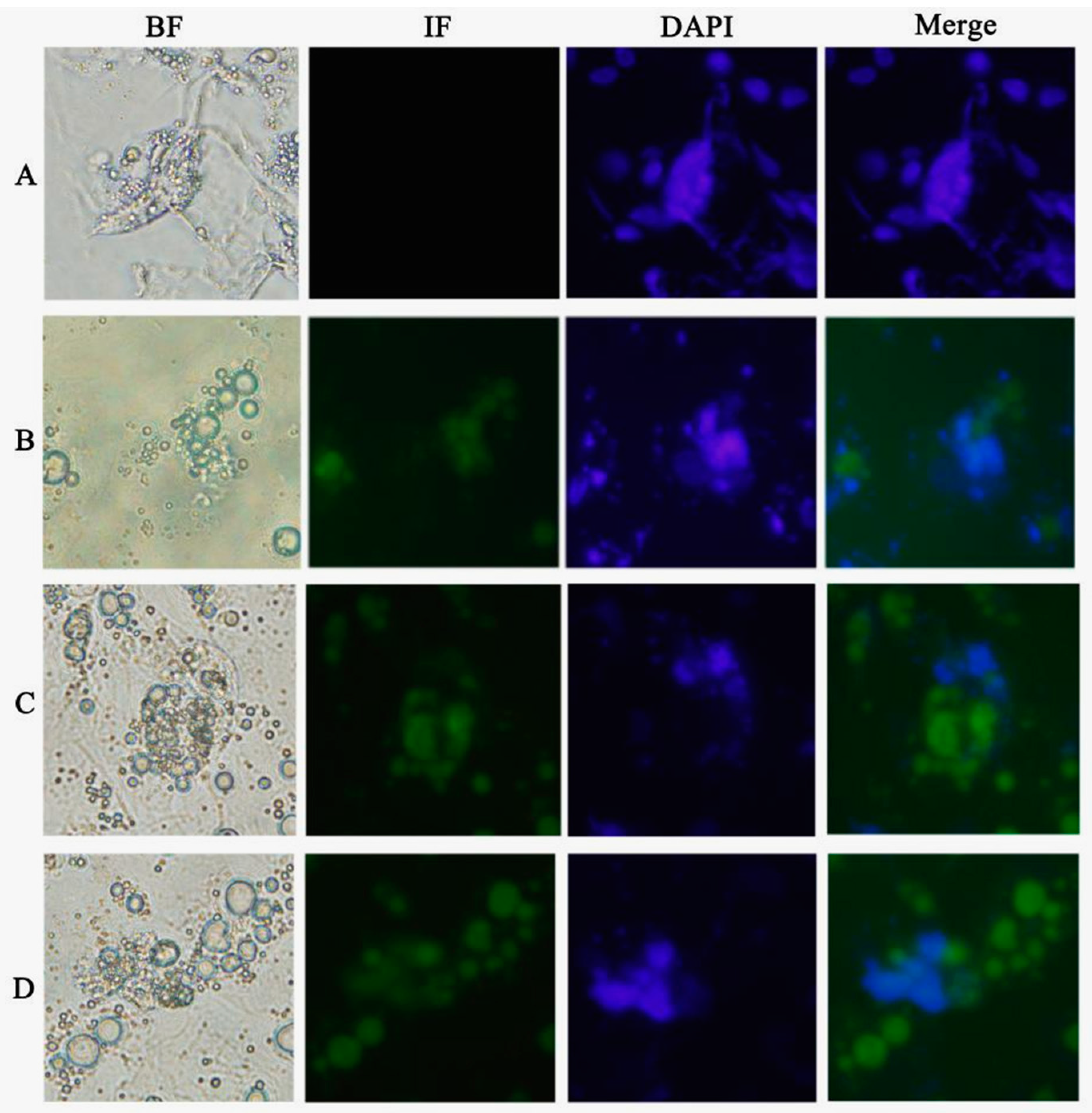
Chicken ESCs were inoculated into 24 wells, approximately 2 × 105 cells per well. ATRA (10−5 mol/L), Am80 (10−6 mol/L), and E2 (1 μg/mL) were used to induce chicken ESC differentiation in vitro. The cells were collected every two days after inducer treatment. The results of each group consisted of three replications.
3.9. Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR)
Total RNA was extracted using the RNeasy kit (Qiagen, Suzhou, China). Reverse transcription into cDNA was performed by Reverse Transcription System (Qiagen) to serve as a template for qRT-PCR. qRT-PCR of the cDNA was performed according to the instructions provided in the fluorescence quantitative PCR kit (Qiagen), using SYBR as the fluorescent reagent and 7500 System fluorescence quantitative instrument (Applied Biosystems, Foster City, CA, USA). Each group had three replications. The data were analyzed in Microsoft Excel using the 2−ΔΔCt relative quantitative method.
4. Discussion
DAZL is one of the DAZ family members encoding an RNA-binding protein, which is also an indispensable control factor during the animal gametogenesis process. To explore the role of the DAZL gene in the specific regulatory mechanism of male reproductive cells, different lengths of the chicken DAZL gene promoter attached to a fluorescent reporter were constructed, and were transfected into DF-1. A Dual-Luciferase® Reporter Assay was performed, showing that the pGL-P3 promoter has the highest activity of the DAZL gene promoter fragments in DF-1 cells. This indicates that there are important regulatory elements in the −382 to −39 bp region of the chicken DAZL gene promoter.
Zhuo
et al. [
13] found that with ectopic expression of the
Dazl gene, both motile-tailed sperm and oocytes could be induced from mouse ESCs. Several other groups [
14,
15,
16,
17] also verified that
Dazl was a master gene controlling germ cell differentiation. However,
DAZL gene research is not comprehensive in chickens, and there is doubt whether specific inducers of the mouse
Dazl gene could be used for directional induction by the chicken ortholog
in vitro. To evaluate the conservation of
DAZL gene function, combining previous research results [
18,
19,
20], RA, ATRA, Am80, BMP4, FSH, E
2, testosterone, and testicular extracts evaluated for induction of
pGL3/334 GC-1 cells.
BMP4 is a multifunctional cytokine, which can induce ESCs to differentiate into MGCs
in vitro. Shi
et al. [
20] found that the expression of
Dazl,
Stra8,
Integrin α6, and
c-kit was enhanced with every 2 days of BMP4 treatment in chicken. In our study, BMP4 treatment did not enhance the activity of the core chicken
DAZL gene promoter. Considering the short treatment time of BMP4 in our study (48 h), the lack of an effect might be because BMP4 does not directly affect the chicken
DAZL gene, but indirectly causes the expression of
DAZL mRNA through interaction with multiple genes. Neither testosterone nor testicular extract had an effect on the chicken
DAZL gene promoter, which is consistent with the results by Sun and colleagues [
12]. Pan
et al. [
19] found that E
2 and FSH could induce mouse ESCs to differentiate into MGCs with high-level expression of mouse
Dazl mRNA on day 7. Our study is consistent with their earlier findings. Additionally E
2 had a more significant effect than FSH in our hands. Retinoic acids, including ATRA and RA, are fat-soluble small molecule metabolites of vitamin A. RA plays an important role in biological development and normal physiological conditions. The compound can mediate cell differentiation, proliferation, apoptosis, and regulate immune function. Kerkis and colleagues found that, 10
−5 mol/L of RA could induce mouse ESCs to differentiate into MGCs positive for
Dazl gene expression. The single factor induction by ATRA and RA in our study showed that both can enhance the expression of the chicken
DAZL gene. Am80 is a new synthetic retinoic acid analogue and specific receptor agonist of RARα. Am80 has a higher induced efficiency than ATRA or RA.
Combining with the prediction of regulatory elements binding sites of chicken
DAZL gene (
Figure S3), a RAR-beta (RARβ) binding site presented in the gene’s core promotor region. This indicated that ATRA and RA could probably promoting chicken
DAZL gene transcription by directly binding this interval. The absence of ER (estrogen receptor) and RARalpha (RARα) binding site indicating that E
2 or Am80 might play its role through other bound transcription factors or by signaling. We also found two AR (androgen receptor) binding sites in this region, ATRA, Am80 or E
2 could also directly binding this interval to paly the role in chicken
DAZL gene transcriptional regulation. We are investigating further about the specific molecular mechanism of chicken
DAZL gene inducer.
To further validate whether the inducers we screened from chicken DF-1 cells could be applied to other species, we also used mouse GC-1 cells for the same test (
Figure S1). The results showed that ATRA, Am80, and E
2 could also significantly increased the activity of the
DAZL gene promoter in GC-1 cells (
Figure S2C). ATRA, Am80, and E
2 can significantly enhance the activity of the chicken
DAZL gene promoter and expression of the encoded DAZL protein. The formation of MGC-like cells was also observed during the induction, indicating that these factors can regulate the minimal chicken
DAZL promoter similar to its mammalian counterparts.