Synthesis, Biological Evaluation and 2D-QSAR Study of Halophenyl Bis-Hydrazones as Antimicrobial and Antitubercular Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry


2.2. Anti-Microbial Activity
2.2.1. Anti-Fungal Activity

| Comp. | R | X or Y | Fungi | Gram Positive Bacteria | Gram Negative Bacteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Af | Sa | Sp | Bs | Pa | St | Kp | Ec | |||
| 14a | Ph- | Cl | NA | NA | NA | NA | NA | NA | NA | NA |
| 14b | Ph- | Br | NA | 13.9 ± 0.44 | 12.4 ± 0.63 | 14.8 ± 0.58 | NA | 15.1 ± 0.58 | 16.4 ± 0.72 | 10.4 ± 0.44 |
| 14c | Ph- | I | 21.2 ± 0.25 | 21.9 ± 0.44 | 23.2 ± 0.37 | 23.9 ± 0.25 | 17.6 ± 0.25 | 20.4 ± 0.63 | 19.2 ± 0.25 | 20.7 ± 0.63 |
| 14d | Ph- | OMe | 11.2 ± 0.32 | 9.4 ± 0.58 | 11.4 ± 0.72 | 13.2 ± 0.72 | NA | 11.6 ± 0.25 | 13.7 ± 0.44 | 10.2 ± 0.58 |
| 14e | 4-MeOC6H4- | F | 19.7 ± 0.32 | 20.1 ± 0.58 | 20.9 ± 0.58 | 20.2 ± 0.32 | NA | 21.3 ± 0.58 | 19.2 ± 0.24 | 20.4 ± 0.58 |
| 14f | 4-MeOC6H4- | Cl | 16.9 ± 0.58 | 17.1 ± 0.58 | 18.8 ± 0.44 | 20.6 ± 0.44 | NA | 17.8 ± 0.63 | 16.4 ± 0.37 | 18.5 ± 0.58 |
| 14g | 4-MeOC6H4- | I | 21.4 ± 0.44 | 20.8 ± 0.58 | 22.3 ± 0.63 | 23.2 ± 0.58 | 15.3 ± 0.25 | 22.4 ± 0.44 | 19.9 ± 0.25 | 21.3 ± 0.44 |
| 14h | 4-MeOC6H4- | Me | 15.6 ± 0.25 | 17.2 ± 0.44 | 17.8 ± 0.37 | 19.9 ± 0.25 | NA | 20.3 ± 0.63 | 18.6 ± 0.25 | 19.7 ± 0.63 |
| 14i | 4-ClC6H4- | H | NA | NA | NA | NA | NA | NA | NA | NA |
| 14j | 4-ClC6H4- | F | NA | NA | NA | NA | NA | NA | NA | NA |
| 14k | 4-ClC6H4- | Me | 15.7 ± 0.58 | 16.9 ± 0.37 | 15.6 ± 0.25 | 17.2 ± 0.58 | NA | 19.1 ± 0.63 | 16.5 ± 0.63 | 18.1 ± 0.72 |
| 14l | 4-ClC6H4- | OMe | 15.3 ± 0.25 | 18.4 ± 0.58 | 16.2 ± 0.63 | 17.3 ± 0.58 | NA | 17.9 ± 0.58 | 18.2 ± 0.72 | 15.8 ± 0.63 |
| 14m | Benzothiazol-2-yl | Cl | NA | 16.2 ± 0.63 | 14.7 ± 0.44 | 15.3 ± 0.44 | NA | 18.6 ± 0.58 | 19.8 ± 0.58 | 18.6 ± 0.44 |
| 14n | 3-Methylbenzofuran-2-yl | Br | NA | NA | NA | NA | NA | NA | NA | NA |
| 16a | Ph- | H | 14.3 ± 0.37 | 17.2 ± 0.63 | 16.8 ± 0.44 | 18.3 ± 0.44 | NA | 16.8 ± 0.25 | 13.4 ± 0.44 | 15.2 ± 0.63 |
| 16b | Ph- | Me | 21.2 ± 0.58 | 22.3 ± 0.63 | 22.8 ± 0.25 | 24.2 ± 0.25 | 18.9 ± 0.25 | 22.9 ± 0.37 | 21.4 ± 0.58 | 20.7 ± 0.63 |
| 16c | Benzothiazol-2-yl | H | 18.1 ± 0.58 | 14.8 ± 0.58 | 13.8 ± 0.58 | 16.3 ± 0.44 | NA | 19.8 ± 0.25 | 18.7 ± 0.44 | 16.1 ± 0.63 |
| 16d | Benzothiazol-2-yl | Me | 19.1 ± 0.63 | 15.7 ± 0.15 | 17.3 ± 0.18 | 19.8 ± 0.22 | NA | 19.7 ± 0.48 | 16.5 ± 0.37 | 17.6 ± 0.25 |
| 17a | Ph- | I | 20.6 ± 0.58 | 21.3 ± 0.58 | 21.9 ± 0.44 | 23.1 ± 0.44 | 17.2 ± 0.58 | 21.3 ± 0.58 | 20.4 ± 0.37 | 20.6 ± 0.58 |
| 17b | 3-Methylbenzofuran-2-yl | Br | 20.4 ± 0.58 | 20.9 ± 0.44 | 21.3 ± 0.58 | 21.9 ± 0.36 | 19.1 ± 0.44 | 22.4 ± 0.58 | 21.4 ± 0.19 | 22.9 ± 0.58 |
| AB | 19.5 ± 0.21 | nt | nt | nt | nt | nt | nt | nt | ||
| CF | nt | 20.0 ± 0.34 | 20.3 ± 0.58 | 20.4 ± 0.14 | 19.2 ± 0.15 | 19.8 ± 0.63 | 19.7 ± 0.12 | 20.3 ± 0.44 | ||
| Comp. | Fungi | Gram Positive Bacteria | Gram Negative Bacteria | |||||
|---|---|---|---|---|---|---|---|---|
| Af | Sa | Sp | Bs | Pa | St | Kp | Ec | |
| 14a | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14b | >125 | 125 | 125 | 62.50 | >125 | 62.50 | 31.25 | 125 |
| 14c | 0.98 | 0.49 | 0.24 | 0.12 | 7.81 | 1.95 | 3.90 | 0.98 |
| 14d | >125 | >125 | 125 | 125 | >125 | 125 | 125 | 125 |
| 14e | 1.95 | 1.95 | 0.98 | 1.95 | >125 | 0.98 | 3.90 | 1.95 |
| 14f | 15.63 | 15.63 | 3.90 | 0.98 | >125 | 7.81 | 15.63 | 3.90 |
| 14g | 0.98 | 0.98 | 0.49 | 0.24 | 62.50 | 0.49 | 1.95 | 0.98 |
| 14h | 31.25 | 31.25 | 7.81 | 1.95 | >125 | 1.95 | 3.90 | 1.95 |
| 14i | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14j | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 14k | 31.25 | 15.63 | 31.25 | 15.63 | >125 | 3.90 | 15.63 | 7.81 |
| 14l | 62.50 | 7.81 | 31.25 | 15.63 | >125 | 7.81 | 7.81 | 31.25 |
| 14m | >125 | 31.25 | 62.50 | 62.50 | >125 | 3.90 | 1.95 | 3.90 |
| 14n | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 16a | 125 | 15.63 | 15.63 | 7.81 | >125 | 15.63 | 125 | 62.50 |
| 16b | 0.49 | 0.49 | 0.24 | 0.12 | 3.90 | 0.24 | 0.98 | 0.98 |
| 16c | 7.81 | 62.50 | 125 | 31.25 | >125 | 3.90 | 3.90 | 31.25 |
| 16d | 3.90 | 31.25 | 15.63 | 1.95 | >125 | 3.90 | 15.63 | 7.81 |
| 17a | 0.98 | 0.98 | 0.49 | 0.24 | 15.63 | 0.98 | 1.95 | 0.98 |
| 17b | 1.95 | 0.98 | 0.98 | 0.98 | 3.90 | 0.49 | 0.98 | 0.24 |
| AB | 1.95 | nt | nt | nt | nt | nt | nt | nt |
| CF | nt | 1.95 | 1.95 | 1.95 | 3.90 | 3.90 | 3.90 | 1.95 |
2.2.2. Antibacterial Activity
2.2.3. Antimycobacterial Activity
| Comp. | I.Z | MIC | C LogP a |
|---|---|---|---|
| 14a | NA | NA | 5.615 |
| 14b | NA | NA | 5.746 |
| 14c | 18.3 ± 0.25 | 7.81 | 6.02 |
| 14d | NA | NA | 4.994 |
| 14e | NA | NA | 5.157 |
| 14f | NA | NA | 5.672 |
| 14g | 18.4 ± 0.25 | 7.81 | 6.077 |
| 14h | NA | NA | 5.442 |
| 14i | NA | NA | 5.615 |
| 14j | NA | NA | 5.779 |
| 14k | NA | NA | 6.064 |
| 14l | NA | NA | 5.672 |
| 14m | NA | NA | 5.780 |
| 14n | NA | NA | 6.687 |
| 16a | NA | NA | 5.504 |
| 16b | 19.4 ± 0.25 | 3.90 | 5.952 |
| 16c | NA | NA | 5.669 |
| 16d | 11.3 ± 0.37 | 125 | 6.118 |
| 17a | 19.3 ± 0.37 | 3.90 | 8.283 |
| 17b | 20.2 ± 0.19 | 1.95 | 8.69 |
| Isoniazide | nt | 0.40 | −0.969 |
| Pyrazinamide | nt | 3.21 | −0.711 |
2.2.4. In Vitro Cytotoxicity
| Compound | IC50 (μM) |
|---|---|
| 16b | 75.3 |
| 17a | >100 |
| 17b | >100 |
| Doxorubicin | 2.3 |
2.3. 2D QSAR Study
2.3.1. Development of QSAR Models
2.3.2. QSAR Study Results


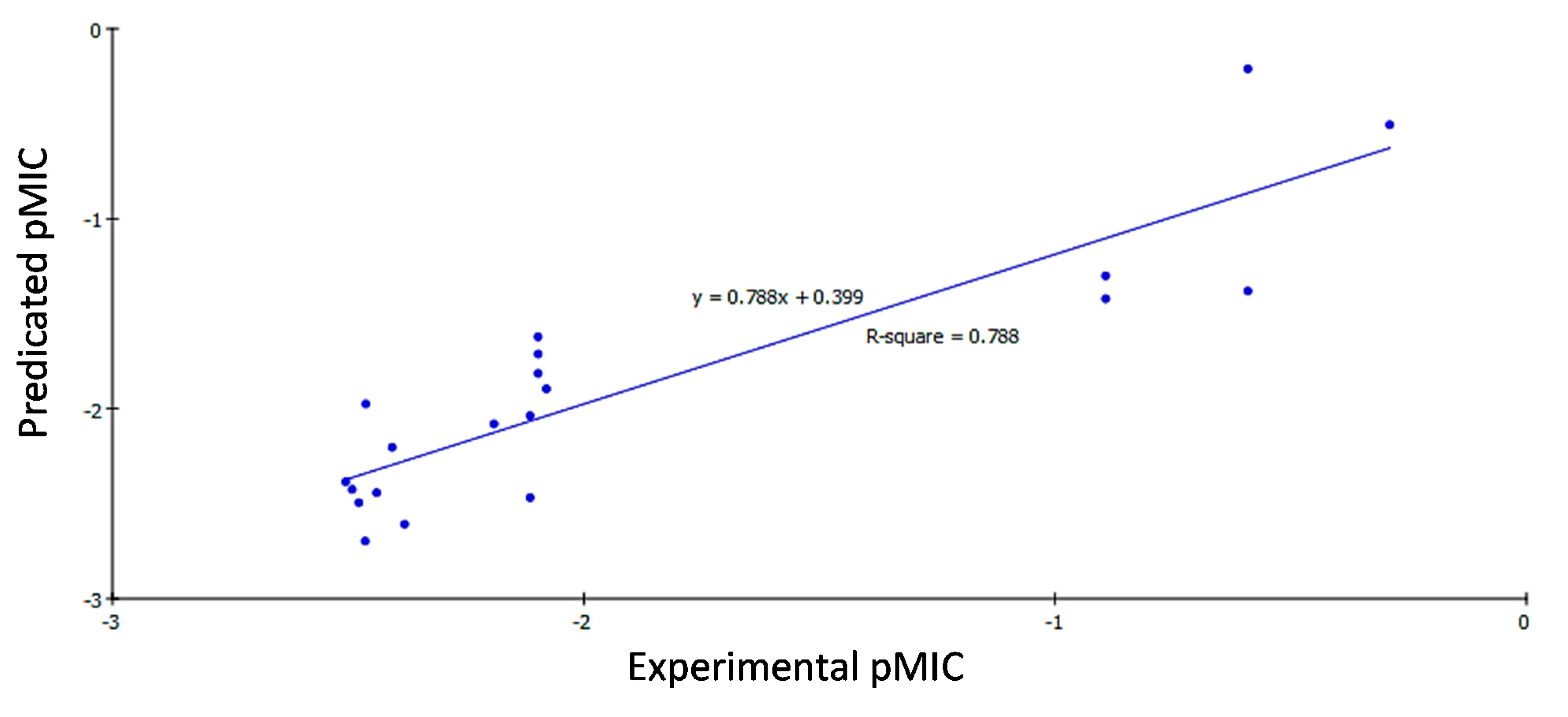
2.3.3. QSAR Validation
| Comp. | Bacillis subtilis | Klebsiella pneumonia | Mycobacterium tuberculosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | Experimental Activity (pMIC) | Predicted Activity (pMIC) | Residual | |
| 14a | −2.1903 | −1.4532 | −0.7371 | 2.3010 | −1.8082 | −0.4929 | −2.0792 | −1.9173 | −0.1619 |
| 14b | −1.7959 | −1.0525 | −0.7434 | −1.4949 | −1.4739 | −0.0209 | −2.0969 | −1.7353 | −0.3616 |
| 14c | 0.9208 | −0.3440 | 1.2648 | −0.5911 | −1.0238 | 0.4328 | −0.8927 | −1.4641 | 0.5715 |
| 14d | −2.0969 | −1.8098 | −0.2871 | −2.0969 | −1.9502 | −0.1467 | −2.4771 | −2.5540 | 0.0769 |
| 14e | −0.2900 | −0.9401 | 0.6501 | −0.5911 | −0.9562 | 0.3651 | −2.4065 | −2.2571 | −0.1494 |
| 14f | 0.0088 | −0.4222 | 0.4309 | −1.1940 | −0.5166 | −0.6774 | −2.4624 | −1.9998 | −0.4626 |
| 14g | 0.6198 | 1.1471 | −0.5273 | −0.2900 | 0.0707 | −0.3607 | −0.8927 | −1.3632 | 0.4706 |
| 14h | −0.2900 | 0.1190 | −0.4091 | −0.5911 | −0.7779 | 0.1869 | −2.0969 | −1.9088 | −0.1881 |
| 14i | −2.3010 | −1.9291 | −0.3719 | −2.3979 | −2.1004 | −0.2975 | −2.5052 | −2.3546 | −0.1505 |
| 14j | −2.2430 | −2.1235 | −0.1195 | −2.1461 | −2.1224 | −0.0237 | −2.4393 | −2.3799 | −0.0595 |
| 14k | −1.1940 | −1.6423 | 0.4483 | −1.1940 | −1.7642 | 0.5703 | −2.4914 | −2.3827 | −0.1087 |
| 14l | −1.1940 | −1.1464 | −0.0475 | −0.8927 | −0.8912 | −0.0015 | −2.4639 | −2.6564 | 0.1925 |
| 14m | −1.7959 | −1.8821 | 0.0863 | −0.2900 | −0.7286 | 0.4386 | −2.3802 | −2.5155 | 0.1352 |
| 14n | −2.1903 | −2.4221 | 0.2318 | −2.1761 | −2.2809 | 0.1048 | −2.1139 | −2.4051 | 0.2912 |
| 16a | −0.8927 | −1.1653 | 0.2727 | −2.0969 | −1.7667 | −0.3302 | −2.1139 | −2.1128 | −0.0012 |
| 16b | 0.9208 | 0.6497 | 0.2711 | 0.0088 | −0.5553 | 0.5641 | −0.5911 | −1.4733 | 0.8823 |
| 16c | −1.4949 | −1.3730 | −0.1218 | −0.5911 | −0.8948 | 0.3037 | −2.1903 | −2.0308 | −0.1595 |
| 16d | −0.2900 | −0.0496 | −0.2405 | −1.1940 | −0.6373 | −0.5567 | −2.0969 | −1.5745 | −0.5225 |
| 17a | 0.6198 | 0.7416 | −0.1218 | −0.2900 | 0.0110 | −0.3010 | −0.5911 | −0.2366 | −0.3545 |
| 17b | 0.0088 | −0.0622 | 0.0710 | 0.0088 | −0.2344 | 0.2432 | −0.2900 | −0.3499 | 0.0599 |
3. Experimental Section
3.1. Chemistry
3.1.1. Synthesis of Hydrazones 14a–n
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-chlorophenyl)propanehydrazonoyl chloride (14a)
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-bromophenyl)propanehydrazonoyl chloride (14b)
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-iodophenyl)propanehydrazonoyl chloride (14c)
(1Z,2E)-2-(2-Benzoylhydrazono)-N-(4-methoxyphenyl)propanehydrazonoyl chloride (14d)
(1Z,2E)-N-(4-Fluorophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propane hydrazonoyl chloride (14e)
(1Z,2E)-N-(4-Chlorophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propane hydrazonoyl chloride (14f)
(1Z,2E)-N-(4-Iodophenyl)-2-(2-(4-methoxybenzoyl)hydrazono)propanehydrazonoyl chloride (14g)
(1Z,2E)-2-(2-(4-Methoxybenzoyl)hydrazono)-N-(p-tolyl)propanehydrazonoyl chloride (14h)
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-phenylpropanehydrazonoyl chloride (14i)
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(4-fluorophenyl)propanehydrazonoyl chloride (14j)
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(p-tolyl)propanehydrazonoyl chloride (14k)
(1Z,2E)-2-(2-(4-Chlorobenzoyl)hydrazono)-N-(4-methoxyphenyl)propanehydrazonoyl chloride (14l)
(1Z,2E)-2-(2-(Benzo[d]thiazole-2-carbonyl)hydrazono)-N-(4-chlorophenyl)propane hydrazonoyl chloride (14m)
(1Z,2E)-N-(4-Bromophenyl)-2-(2-(3-methylbenzofuran-2-carbonyl)hydrazono)propane hydrazonoyl chloride (14n)
3.1.2. Synthesis of Compounds 16a–d
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-(phenylsulfonyl)propan-2-ylidene) benzohydrazide (16a)
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-tosylpropan-2-ylidene)benzohydrazide (16b)
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-(phenylsulfonyl)propan-2-ylidene) benzo [d]thiazole-2-carbohydrazide (16c)
N'-((1Z,2E)-1-(2-(4-Chlorophenyl)hydrazono)-1-tosylpropan-2-ylidene)benzo[d] thiazole-2-carbohydrazide (16d)
3.1.3. Synthesis of Compounds 17a,b
N'-((1Z,2E,3E)-1-(2-(4-Iodophenyl)hydrazono)-4-phenyl-1-(piperidin-1-yl)but-3-en-2-ylidene)benzohydrazide (17a)
N'-((1Z,2E,3E)-1-(2-(4-Bromophenyl)hydrazono)-4-phenyl-1-(piperidin-1-yl)but-3-en-2-ylidene)-3-methylbenzofuran-2-carbohydrazide (17b)
3.2. Biological Evaluation
3.2.1. Antimicrobial Activity
3.2.2. Minimum Inhibitory Concentration
3.2.3. Antimycobacterial Activity
3.2.4. In Vitro Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 11, 895–910. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Ritter, T.K.; Wong, C. Carbohydrate-based antibiotics: A new approach to tackling the problem of resistance. Angew. Chem. Int. Ed. 2001, 40, 3508–3533. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2013; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.C.; Plumley, K.V.; Laughon, B.E. The evolution of extensively drug resistant tuberculosis (XDR-TB): History, status and issues for global control. Infect. Disord. Drug Targets 2007, 7, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Benatar, S.R. Extensively drug resistant tuberculosis—Problem will get worse in South Africa unless poverty is alleviated. Br. Med. J. 2006, 333, 705. [Google Scholar] [CrossRef]
- Lawn, S.D.; Wilkinson, R. Extensively drug resistant tuberculosis-a serious wake-up call for global health. Br. Med. J. 2006, 333, 559–560. [Google Scholar] [CrossRef]
- Dahle, U.R. Extensively drug resistant tuberculosis-beware patients lost to follow-up. Br. Med. J. 2006, 333, 705. [Google Scholar] [CrossRef]
- Gandhi, N.R.; Moll, A.; Sturm, A.W.; Pawinski, R.; Govender, T.; Lalloo, U.; Zeller, K.; Andrews, J.; Friedland, G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006, 368, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Manissero, D.; Fernandez, K. Extensive drug-resistant TB: A threat for Europe? Eurosurveillance 2006, 11, E060928. [Google Scholar] [PubMed]
- Küçükgüzel, S.G.; Oruç, E.E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Ragavendran, J.V.; Sriram, D.; Patel, S.K.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Saini, V. Synthesis, characterization, and computational studies on phthalic anhydride-based benzylidene-hydrazide derivatives as novel, potential anti-inflammatory agents. Med. Chem. Res. 2014, 23, 2676–2689. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N.; et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar]
- Pavan, F.R.; da S. Maia, P.I.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Vavøíková, E.; Polanc, S.; Horváti, K.; Bosze, S.; Stolaríková, J.; Vávrová, K.; Vinsová, J. New fluorine-containing hydrazones active against MDR-tuberculosis. Bioorg. Med. Chem. Lett. 2005, 15, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, E.; Karhumaki, E.; Langshaw, J. Carbohydrazones of substituted salicylaldehydes as potential lead compounds for the development of narrow-spectrum antimicrobials. J. Biosci. 2007, 62, 483–486. [Google Scholar]
- Torres, E.; Moreno, E.; Ancizu, S.; Barea, C.; Galiano, S.; Aldana, I.; Monge, A.; Pérez-Silanes, S. New 1,4-di-N-oxide-quinoxaline-2-ylmethylene isonicotinic acid hydrazide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. Lett. 2011, 21, 3699–3703. [Google Scholar]
- Tavares, L.C.; Chiste, J.J.; Santos, M.B.; Penna, T. Synthesis and biological activity of nifuroxazide and analogs. II. Boll. Chim. Farm. 1999, 183, 432–436. [Google Scholar]
- More, U.A.; Joshi, S.D.; Aminabhavi, T.M.; Gadad, A.K.; Nadagouda, M.N.; Kulkarni, V.H. Design, synthesis, molecular docking and 3D-QSAR studies of potent inhibitors of enoyl-acyl carrier protein reductase as potential antimycobacterial agents. Eur. J. Med. Chem. 2014, 71, 199–218. [Google Scholar] [CrossRef] [PubMed]
- De Logu, A.; Onnis, V.; Saddi, B.; Congiu, C.; Schivo, M.L.; Cocco, M.T. Activity of a new class of isonicotinoylhydrazones used alone and in combinatin with isoniazid, rifampicin, ethambutol, para-aminosalicylic acid and clofazimine against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2002, 49, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Kang, H.J.-O.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D.; et al. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 54, 4169–4178. [Google Scholar]
- Aboul-Fadl, T.; Mohammed, F.A.; Hassan, E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.G.; Limbird, L.E. Goodman & Gilman’s. In The Pharmacological Basis of Therapeutics, 10th ed.; McGraw-Hill Hardman: New York, NY, USA, 2001. [Google Scholar]
- Shaharyar, M.; Siddiqui, A.A.; Ali, M.A.; Sriram, D.; Yogeeswari, P. Synthesis and in vitro antimycobacterial activity of N1-nicotinoyl-3-(4'-hydroxy-3'-methyl phenyl)-5-[(sub)phenyl]-2-pyrazolines. Bioorg. Med. Chem. Lett. 2006, 16, 3947–3949. [Google Scholar] [CrossRef] [PubMed]
- Moussa, Z.; El-Sharief, M.A.; El-Sharief, A.M. Synthesis and characterization of new types of halogenated and alkylated imidazolidineiminothiones and a comparative study of their antitumor, antibacterial, and antifungal activities. Eur. J. Med. Chem. 2011, 46, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Telvekar, V.N.; Bairwa, V.K.; Satardekar, K.; Bellubi, A. Novel 2-(2-(4-aryloxy benzylidene)hydrazinyl)benzothiazole derivatives as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2011, 22, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ioerger, T.R.; Sacchettini, J.C. Discovery of novel nitrobenzothiazole inhibitors for Mycobacterium tuberculosis ATP phosphoribosyl transferase (HisG) through virtual screening. J. Med. Chem. 2008, 51, 5984–5992. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.A.; Massabni, A.C.; Castellano, E.E.; Costa, L.A.S.; Leite, C.Q.F.; Pavan, F.R.; Cuin, A. A broad study of two new promising antimycobacterial drugs: Ag(I) and Au(I) complexes with 2-(2-thienyl)benzothiazole. Polyhedron 2012, 38, 291–296. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, F.; Jiang, X.; Zhang, W.; Liu, J.; Liu, W.; Fu, L. Synthesis and antimicrobial evaluation of 3-methanone-6-substituted benzofuran derivatives. Eur. J. Med. Chem. 2012, 54, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Abdel-Wahab, B.F.; Badria, F.A. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(Piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl)hydrazono]-4-(ary1)but-3-enes. Arch. Pharm. 2010, 343, 152–159. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Mekawey, A.A.I. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur. J. Med. Chem. 2009, 44, 4985–4997. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Convenient synthesis and antimicrobial activity of new 3-substituted 5-(Benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008, 341, 734–739. [Google Scholar] [CrossRef]
- Ghabbour, H.A.; Qabeel, M.M.; Eldehna, W.M.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Design, synthesis, and molecular docking of 1-(1-(4-chlorophenyl)-2-(phenylsulfonyl)ethylidene)-2-phenylhydrazine as potent nonazole anticandidal agent. J. Chem. 2014, 2014, 154357. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A.; Elaasser, M.M.; Abdel-Aziz, M.M. Improvement of antibacterial activity of some sulfa drugs through linkage to certain phthalazin-1(2H)-one scaffolds. Eur. J. Med. Chem. 2014, 85, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Devakaram, V.R. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006, 14, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S.; Farghaly, T.A. Reactions of hydrazonoyl halides with heterocyclic thiones. Convenient methodology for heteroannulation, synthesis of spiroheterocycles and heterocyclic ring transformation. Arkivoc 2008, i, 18–64. [Google Scholar]
- Padmavathi, V.; Reddy, S.N.; Mahesh, K. Synthesis, antimicrobial and antioxidant activities of sulfone linked bis heterocycles-pyrazolyl oxadiazoles and pyrazolyl thiadiazole. Chem. Pharm. Bull. 2009, 57, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, A.; Venkatesh, B.C.; Padmavathi, V.; Padmaja, A.; Kondaiah, P.; Krishna, N.S. Synthesis, antimicrobial and cytotoxic activities of sulfone linked bis-heterocycles. Eur. J. Med. Chem. 2012, 54, 605–614. [Google Scholar]
- Dagenais, T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009, 3, 447–465. [Google Scholar] [CrossRef]
- Morikawa, H.; Tomishima, M.; Kayakiri, N.; Araki, T.; Barrett, D.; Akamatsu, S.; Matsumoto, S.; Uchida, S.; Nakai, T.; Takeda, S.; et al. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg. Med. Chem. Lett. 2014, 24, 1172–1175. [Google Scholar]
- Yamasaki, M.; Harada, E.; Tamura, Y.; Lim, S.; Ohsuga, T.; Yokoyama, N.; Morishita, K.; Nakamura, K.; Ohta, H.; Takiguchi, M. In vitro and in vivo safety and efficacy studies of amphotericin B on Babesia gibson. Vet. Parasitol. 2014, 205, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A.; Hooper, D.C. Review of the Quinolone Family; Springer US: New York, NY, USA, 2012. [Google Scholar]
- Maccari, R.; Ottana, R.; Vigorita, M.G. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorg. Med. Chem. Lett. 2005, 15, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.O.; Qandil, A.M.; Zaki, D.D.; Al Damen, M.A. Ligand-based assessment of factor Xa binding site flexibility via elaborate pharmacophore exploration and genetic algorithm-based QSAR modeling. Eur. J. Med. Chem. 2005, 40, 701–727. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.T.; Jurs, P.C. Development and use of charge partial surface area structural descriptors in computer-assisted quantitative structure-property relationship studies. Anal. Chem. 1990, 62, 2323–2329. [Google Scholar] [CrossRef]
- Rohrbaugh, R.H.; Jurs, P.C. Descriptions of molecular shape applied in studies of structure/activity and structure/property relationships. Anal. Chim. Acta 1987, 199, 99–109. [Google Scholar] [CrossRef]
- Irobi, O.N.; Moo-Young, M.; Anderson, W.A. Antimicrobial activity of annatto (Bixa orellana) extract. Pharm. Biol. 1996, 34, 87–90. [Google Scholar] [CrossRef]
- Urzua, A.; Caroli, M.; Vasquez, L.; Mendoza, L.; Wilkens, M.; Tojo, E. Antimicrobial study of the resinous exudate and of diterpenoids isolated from Eupatorium salvia (Asteraceae). J. Ethnopharmacol. 1998, 62, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Aziz, H.A.; Eldehna, W.M.; Fares, M.; Al-Rashood, S.T.A.; Al-Rashood, K.A.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, Biological Evaluation and 2D-QSAR Study of Halophenyl Bis-Hydrazones as Antimicrobial and Antitubercular Agents. Int. J. Mol. Sci. 2015, 16, 8719-8743. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048719
Abdel-Aziz HA, Eldehna WM, Fares M, Al-Rashood STA, Al-Rashood KA, Abdel-Aziz MM, Soliman DH. Synthesis, Biological Evaluation and 2D-QSAR Study of Halophenyl Bis-Hydrazones as Antimicrobial and Antitubercular Agents. International Journal of Molecular Sciences. 2015; 16(4):8719-8743. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048719
Chicago/Turabian StyleAbdel-Aziz, Hatem A., Wagdy M. Eldehna, Mohamed Fares, Sara T. A. Al-Rashood, Khalid A. Al-Rashood, Marwa M. Abdel-Aziz, and Dalia H. Soliman. 2015. "Synthesis, Biological Evaluation and 2D-QSAR Study of Halophenyl Bis-Hydrazones as Antimicrobial and Antitubercular Agents" International Journal of Molecular Sciences 16, no. 4: 8719-8743. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048719







