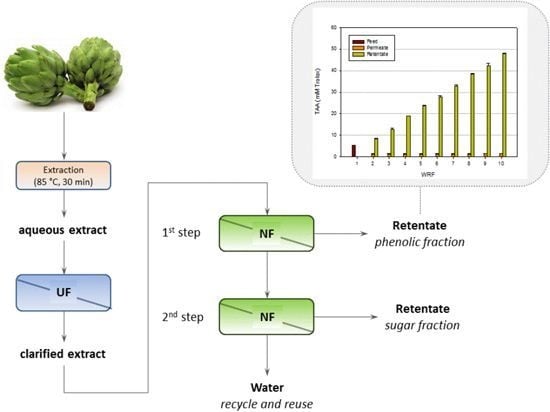
A Two-Step Nanofiltration Process for the Production of Phenolic-Rich Fractions from Artichoke Aqueous Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experiments with the NP030 Membrane
| Parameters | Permeate |
|---|---|
| pH | 4.1 ± 0.1 |
| TSS (°Brix) | 2.4 ± 0.1 |
| Suspended solids (%) | n.d. |
| Glucose (mg/L) | 642.0 ± 3.0 |
| Fructose (mg/L) | 577.0 ± 4.0 |
| Sucrose (mg/L) | 745.0 ± 1.0 |
| Total polyphenols (mg/L gallic acid) | 479.1 ± 2.9 |
| Chlorogenic acid (mg/L) | 176.3 ± 2.4 |
| Cynarin (mg/L) | 110.6 ± 0.8 |
| Apigenin 7-O-glucoside (mg/L) | 101.8 ± 0.3 |
| TAA (mM trolox) | 5.33 ± 0.05 |
2.1.1. Experiments according to the Total Recycle Configuration
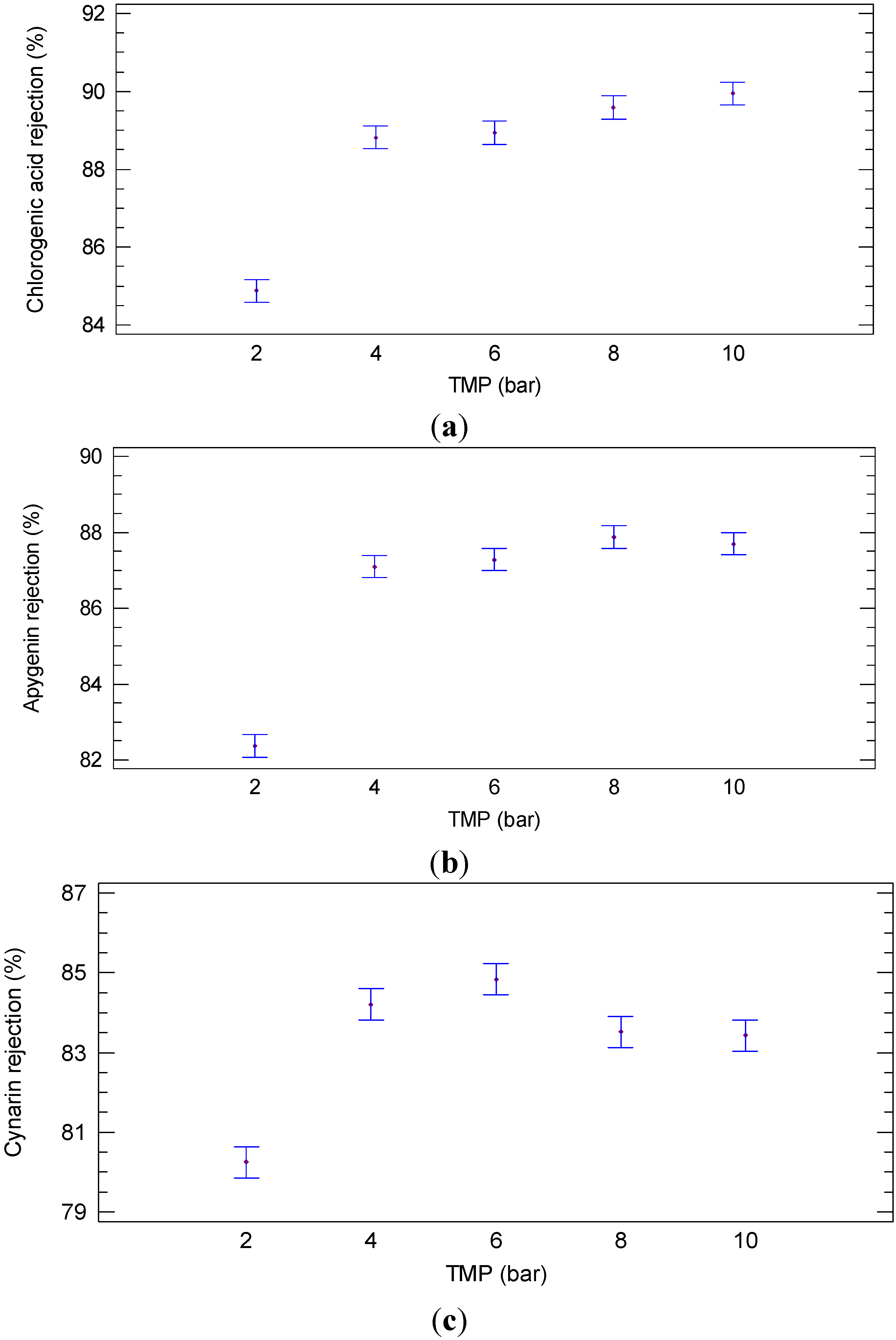
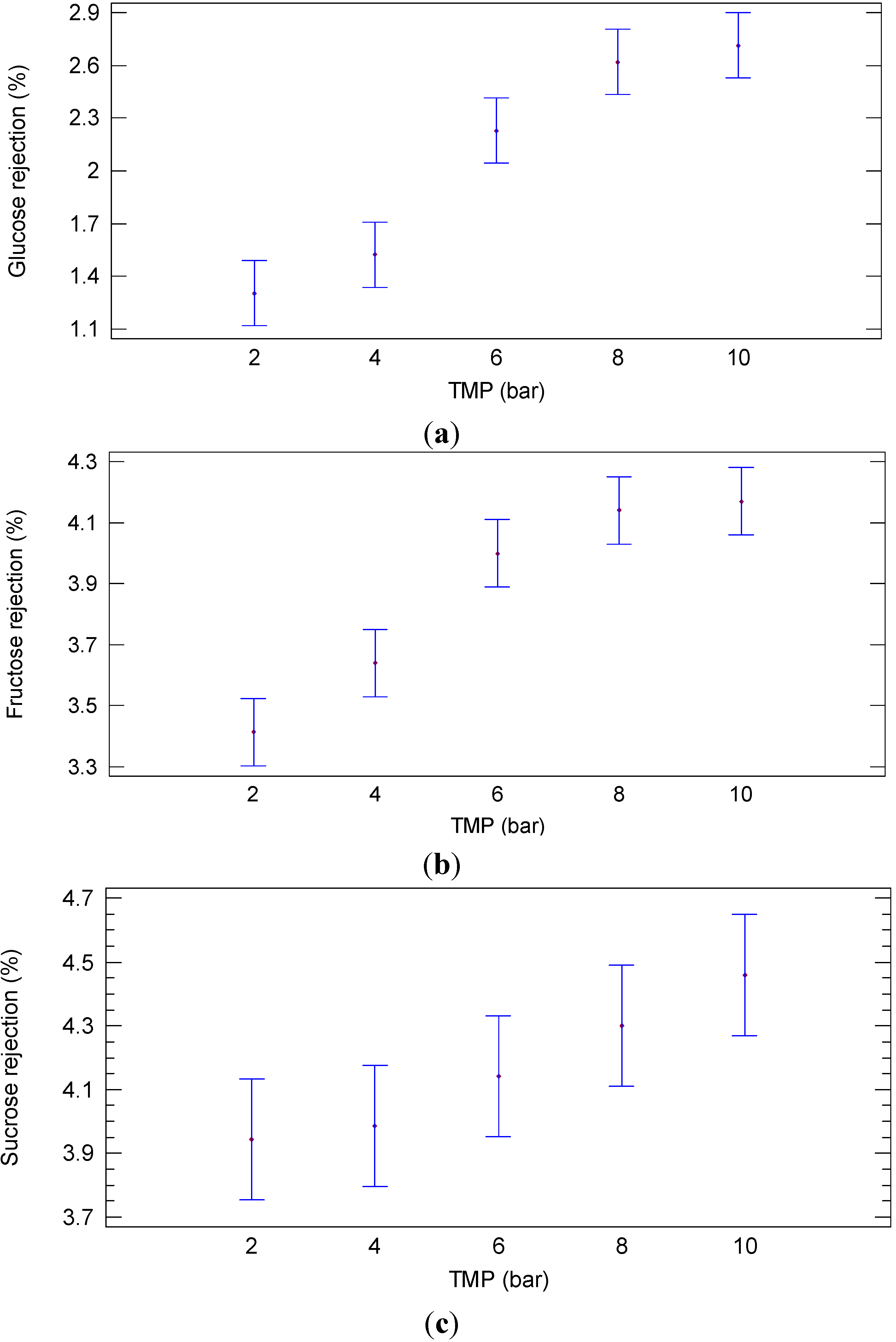

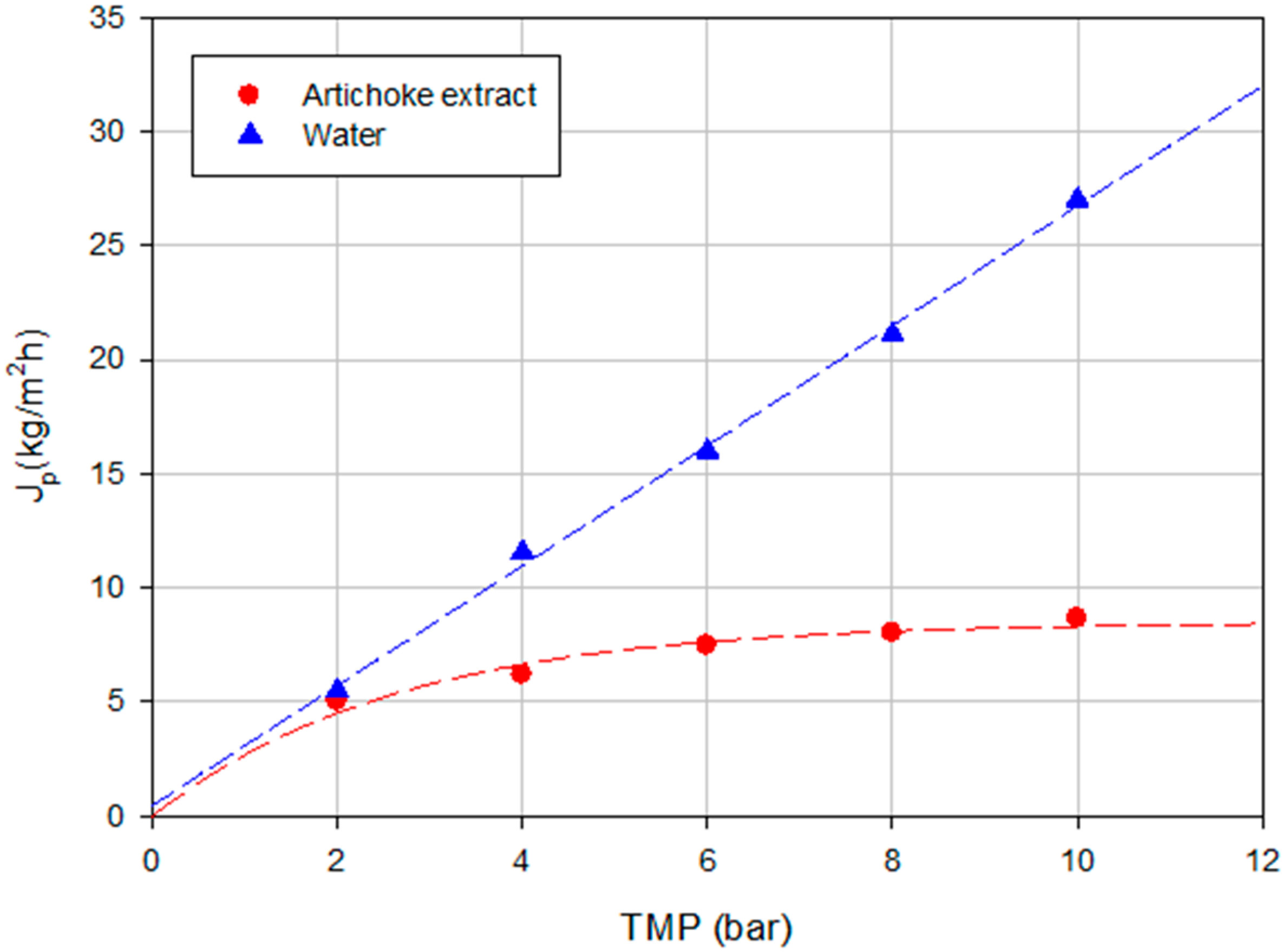
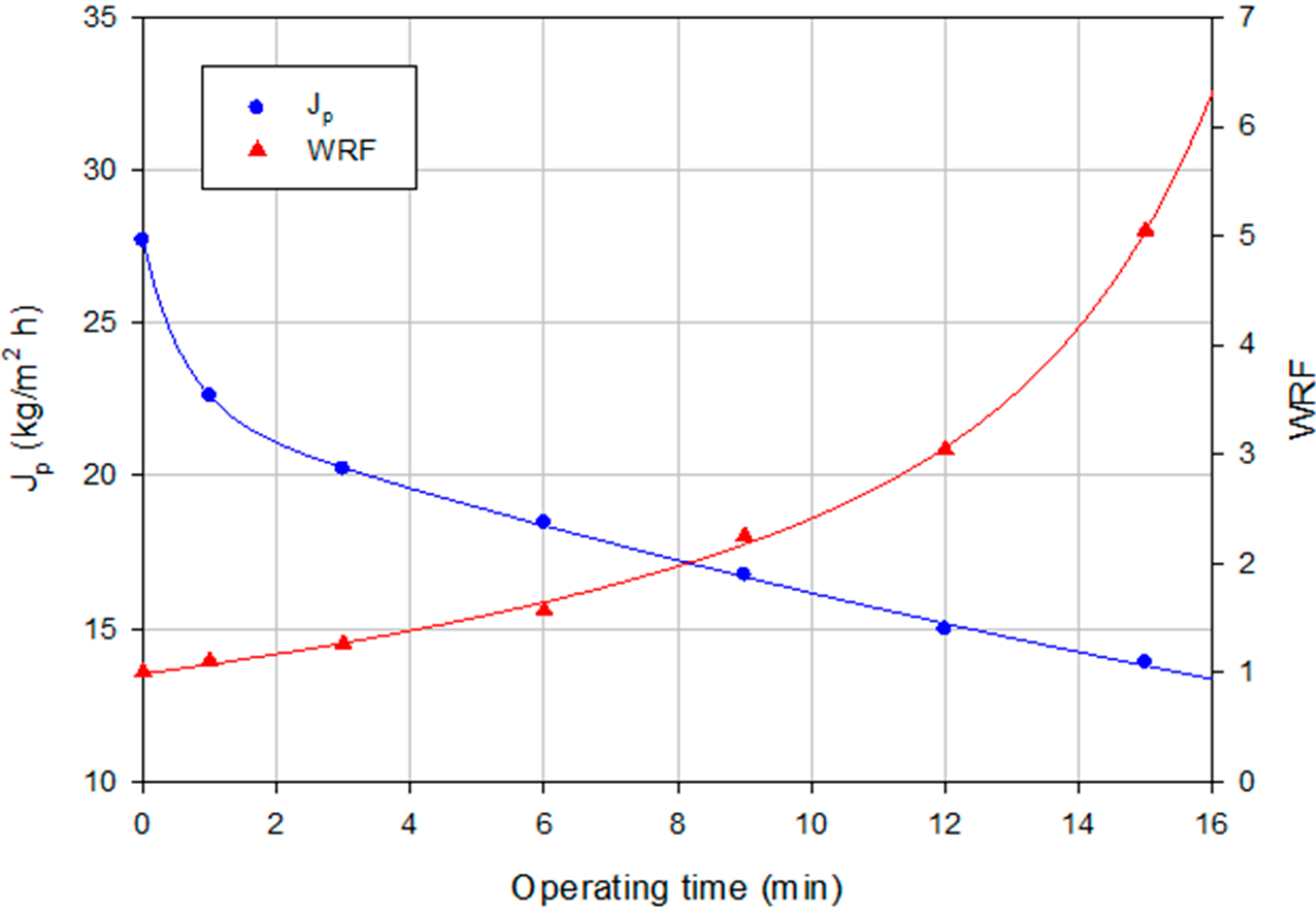
2.1.2. Experiments according to the Batch Concentration Configuration

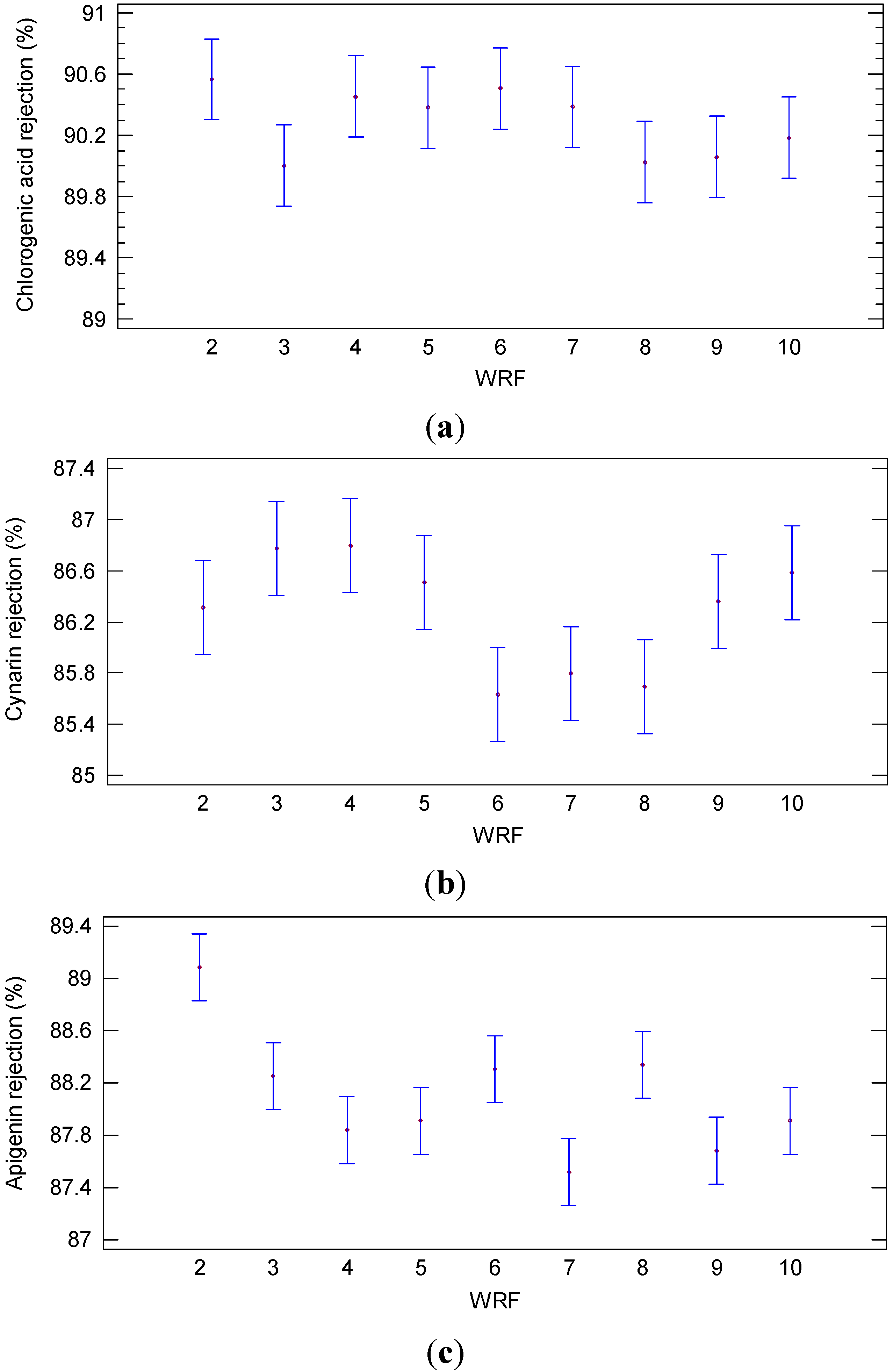
| Sample | WRF | Chlorogenic Acid (mg/L) | Cynarin (mg/L) | Apigenin (mg/L) |
|---|---|---|---|---|
| Feed | - | 168.00 ± 0.55 | 103.00 ± 0.60 | 96.20 ± 0.60 |
| Permeate | 2 | 15.85 ± 0.63 | 14.10 ± 0.50 | 10.50 ± 0.30 |
| 3 | 16.80 ± 0.84 | 13.62 ± 0.17 | 11.30 ± 0.10 | |
| 4 | 16.04 ± 0.76 | 13.60 ± 0.90 | 11.70 ± 0.10 | |
| 5 | 16.17 ± 0.38 | 13.90 ± 0.70 | 11.65 ± 0.17 | |
| 6 | 15.95 ± 0.35 | 14.80 ± 0.60 | 11.25 ± 0.45 | |
| 7 | 16.15 ± 0.43 | 14.63 ± 0.21 | 12.01 ± 0.39 | |
| 8 | 16.76 ± 0.62 | 14.74 ± 0.62 | 11.22 ± 0.68 | |
| 9 | 16.70 ± 0.35 | 14.05 ± 0.80 | 11.85 ± 0.25 | |
| 10 | 16.49 ± 0.53 | 13.82 ± 0.37 | 11.65 ± 0.25 | |
| Retentate | 2 | 201.70 ± 2.40 | 172.90 ± 2.70 | 146.40 ± 1.90 |
| 3 | 257.15 ± 1.62 | 213.4 ± 1.10 | 198.10 ± 0.50 | |
| 4 | 347.10 ± 1.55 | 304.45 ± 1.95 | 259.45 ± 0.85 | |
| 5 | 452.60 ± 0.84 | 414.4 ± 1.80 | 347.25 ± 1.05 | |
| 6 | 512.70 ± 0.98 | 482.90 ± 2.70 | 414.55 ± 2.05 | |
| 7 | 603.25 ± 1.34 | 577.25 ± 0.95 | 505.25 ± 1.00 | |
| 8 | 702.80 ± 0.56 | 682.80 ± 2.60 | 647.05 ± 0.25 | |
| 9 | 917.30 ± 1.55 | 766.90 ± 1.50 | 703.85 ± 0.45 | |
| 10 | 1224.00 ± 2.19 | 898.05 ± 0.55 | 814.35 ± 2.05 |


2.2. Experiments with the Desal DK Membrane
| Parameters | Feed |
|---|---|
| Glucose (mg/L) | 617.15 ± 0.43 |
| Fructose (mg/L) | 547.75 ± 0.70 |
| Sucrose (mg/L) | 708.70 ± 0.70 |
| Chlorogenic acid (mg/L) | 16.49 ± 0.53 |
| Cynarin (mg/L) | 13.82 ± 0.38 |
| Apigenin 7-O-glucoside (mg/L) | 11.62 ± 0.22 |
| TAA (mM trolox) | 1.39 ± 0.01 |
2.2.1. Experiments according to the Total Recycle Configuration
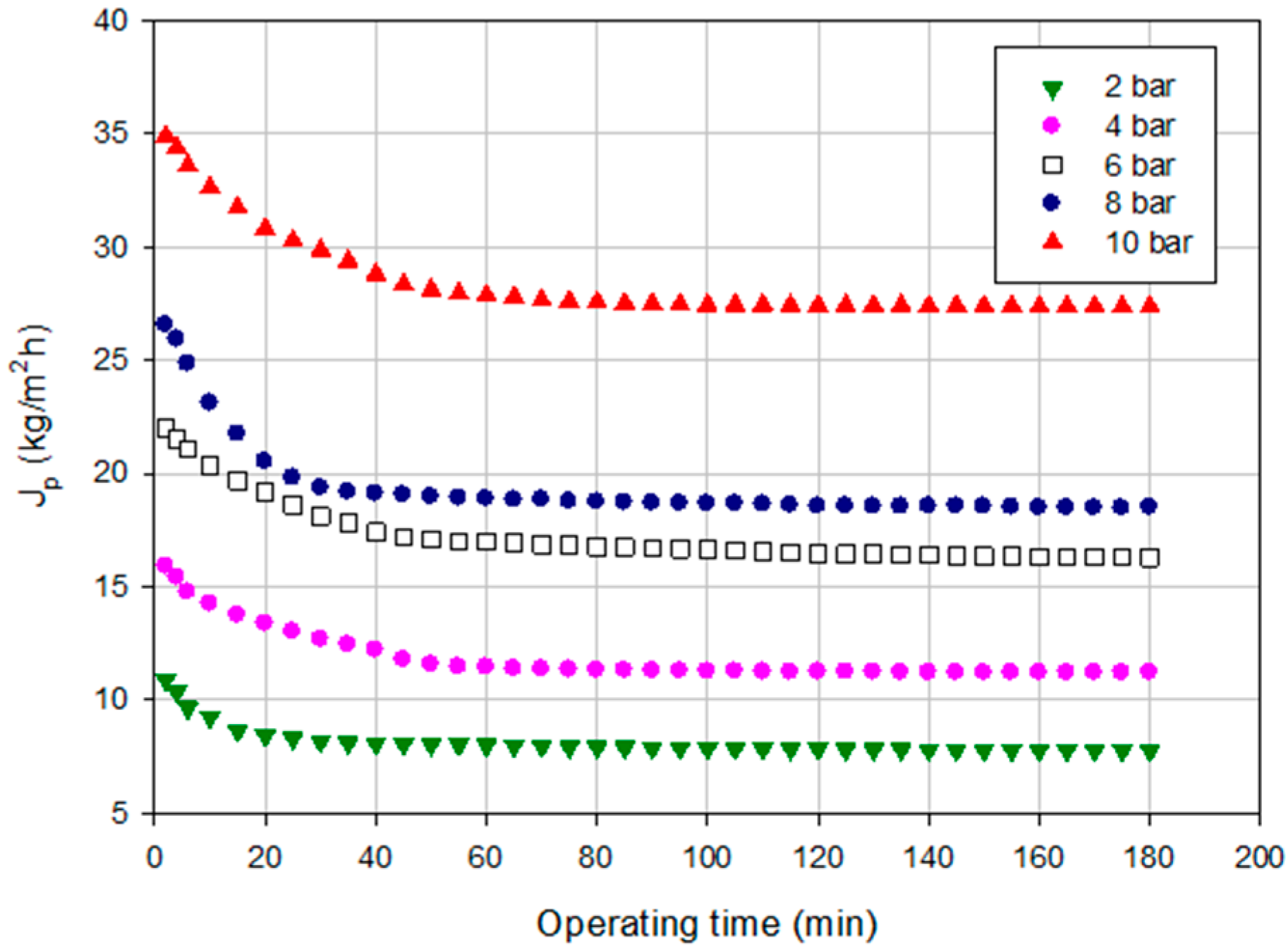
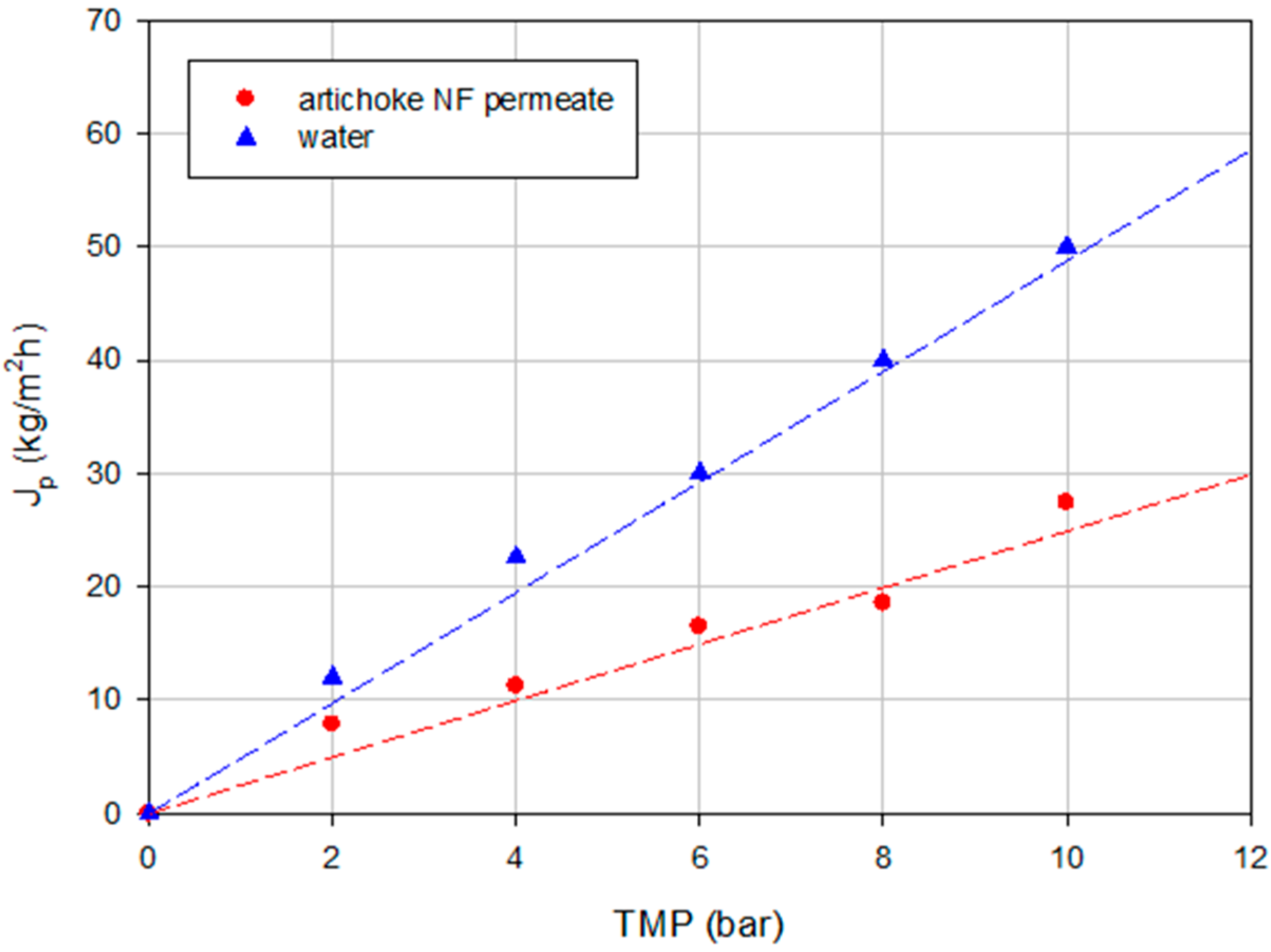
2.2.2. Experiments according to the Batch Concentration Configuration
| Sample | WRF | Sugars | Phenolic Compounds | ||||
|---|---|---|---|---|---|---|---|
| Glucose (mg/L) | Fructose (mg/L) | Sucrose (mg/L) | Chlorogenic Acid (mg/L) | Cynarin (mg/L) | Apigenin-7-O-Glucoside (mg/L) | ||
| Feed | - | 631.0 ± 2.2 | 569.1 ± 3.1 | 737.7 ± 1.7 | 14.5 ± 0.3 | 12.5 ± 0.1 | 9.6 ± 0.1 |
| Permeate | 2–5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Retentate | 2 | 903.0 ± 1.4 | 815.0 ± 1.3 | 1113.5 ± 1.0 | 22.5 ± 0.3 | 17.5 ± 1.1 | 14.6 ± 0.2 |
| 3 | 1521.0 ± 0.1 | 1235.5 ± 1.0 | 1814.5 ± 1.9 | 36.1 ± 0.7 | 27.7 ± 0.5 | 21.8 ± 0.5 | |
| 4 | 2094.7 ± 4.6 | 1814.4 ± 2.0 | 2613.5 ± 1.2 | 45.5 ± 1.0 | 37.5 ± 0.9 | 30.4 ± 0.2 | |
| 5 | 2715.2 ± 1.5 | 2221.9 ± 1.6 | 3113.8 ± 1.0 | 67.5 ± 1.2 | 55.5 ± 1.3 | 43.6 ± 1.0 | |
3. Experimental Section
3.1. Feed Solution
3.2. NF Equipment and Procedures
3.3. Analytical Methods
3.3.1. Total Phenols
3.3.2. Polyphenols
3.3.3. Sugars
3.3.4. Total Antioxidant Activity (TAA)
3.4. Data Analysis
4. Conclusions
- -
- a retentate fraction from the first NF step enriched in phenolic compounds with high antioxidant activity (the ABTS radical scavenging capacity was increased by 9 times in comparison with the initial feed) of interest for nutraceutical or food applications;
- -
- a retentate fraction from the second NF step enriched in sugar compounds;
- -
- a clear permeate from the second NF step free of sugars and phenolic compounds which can be reused for irrigation or recycled in the artichoke processing industry.
Author Contributions
Conflicts of Interest
References
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Bianco, V.V. Present situation and future potential of artichoke in the Mediterranean basin. Acta Hortic. 2005, 681, 39–55. [Google Scholar]
- Pandino, P.; Lombardo, S.; Mauromicale., G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Food 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Lutz, M.; Henríquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Pandino, P.; Lombardo, S.; Mauro, P.; Mauromicale, G. Variation in polyphenols profile and heat morphology among clones of globe artichoke selected from a lantrace. Sci. Hortic. 2012, 138, 259–265. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food. Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escrig, A.; Dragsted, L.O.; Daneshvar, B.; Pulido, R.; Saura-Calixto, F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. J. Agric. Food. Chem. 2003, 51, 5540–5545. [Google Scholar]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Food 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
- Guida, V.; Ferrari, G.; Pataro, G.; Chambery, A.; di Maro, A.; Parente, A. The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT Food Sci. Technol. 2013, 53, 569–579. [Google Scholar] [CrossRef]
- Megias, M.D.; Martinez-Teruel, A.; Hérnandez, M.R. Potential environmental impact of effluents from the artichoke (Cynara scolymus) byproduct ensiling process using additives. J. Agric. Food Chem. 1999, 47, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.; Megias, M.D.; Madrid, J.; Martinez-Teruel, A.; Hernandez, F.; Oliva, J. Evaluation of the phytosanitary, fermentative and nutritive characteristics of the silage made from crude artichoke (Cynara scolymus L.) by-product feeding for ruminants. Small Ruminant Res. 2007, 70, 292–296. [Google Scholar] [CrossRef]
- Llorach, R.; Espin, J.C.; Tomas-Barberan, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) by products as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar]
- Conde, E.; Diaz-Reinoso, B.; Gonzáles-Muños, M.J.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery and concentration of antioxidants from industrial effluents and from processing streams of underutilized vegetal biomass. Food Public Health 2013, 3, 69–91. [Google Scholar]
- Galanakis, C. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Shari, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant material: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. Extraction of natural antioxidants from plant food. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Rizvi, S.S.H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 506–594. [Google Scholar]
- Li, J.; Chase, A.H. Application of membrane techniques for purification of natural products. Biotechnol. Lett. 2010, 32, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Akin, O.; Temelli, F.; Sefa, K. Membrane applications in functional foods and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2012, 52, 347–371. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Salehi, F. Current and future applications for nanofiltration technology in the food processing. Food Bioprod. Process. 2014, 92, 161–177. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Amar, R.B.; Belleville, M.P. Fractionation of a tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013, 108, 28–36. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar]
- Hinkova, A.; Zidova, P.; Pour, V.; Bubnik, Z.; Henke, S.; Salova, A.; Kadlec, P. Potential of membrane separation processes in cheese whey fractionation and separation. Proced. Eng. 2012, 42, 1425–1436. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia-Castello, E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. Recovery of phenolic compounds from orange press liquor by nanofiltration. Food. Bioprod. Process. 2012, 90, 867–874. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Rarajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Gupta. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar]
- Bacchin, P.; Aimar, P.; Field, R.W. Critical and sustainable fluxes: Theory, experiments and applications. J. Membr. Sci. 2006, 281, 42–69. [Google Scholar] [CrossRef] [Green Version]
- Aguiar Prudêncio, A.P.; Prudêncio, E.S.; Castanho Amboni, R.D.M.; Negrão Murakami, A.N.; Maraschin, M.; Cunha Petrus, J.C.; Ogliari, P.J.; Leite, R.S. Phenolic composition and antioxidant activity of the aqueous extract of bark from residues from mate tree(Ilex paraguariensis St. Hil.) bark harvesting concentrated by nanofiltration. Food Bioprod. Process. 2012, 90, 399–405. [Google Scholar] [CrossRef]
- Tylkowski, B.; Tsibranska, I.; Kochanov, R.; Peev, G.; Giamberini, M. Concentration of biologically active compounds extracted from Sideritis. ssp. L. by nanofiltration. Food Bioprod. Process. 2011, 89, 307–314. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- De Fernandez, M.D.; SotoVargas, V.C.; Silva, M.F. Phenolic compounds and antioxidant capacity of monovarietal olive oils produced in argentina. J. Am. Oil Chem. Soc. 2014, 91, 2021–2033. [Google Scholar] [CrossRef]
- Ma, Y.R.; Huang, H.H. Characterisation and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Cunha Petrus, J.C.; Hubinger, M.D. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J. Food Eng. 2010, 96, 533–539. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 243, 279–298. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassano, A.; Conidi, C.; Ruby Figueroa, R.; Castro Muñoz, R. A Two-Step Nanofiltration Process for the Production of Phenolic-Rich Fractions from Artichoke Aqueous Extracts. Int. J. Mol. Sci. 2015, 16, 8968-8987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048968
Cassano A, Conidi C, Ruby Figueroa R, Castro Muñoz R. A Two-Step Nanofiltration Process for the Production of Phenolic-Rich Fractions from Artichoke Aqueous Extracts. International Journal of Molecular Sciences. 2015; 16(4):8968-8987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048968
Chicago/Turabian StyleCassano, Alfredo, Carmela Conidi, René Ruby Figueroa, and Roberto Castro Muñoz. 2015. "A Two-Step Nanofiltration Process for the Production of Phenolic-Rich Fractions from Artichoke Aqueous Extracts" International Journal of Molecular Sciences 16, no. 4: 8968-8987. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16048968