Aminolevulinic Acid-Mediated Photodynamic Therapy of Human Meningioma: An in Vitro Study on Primary Cell Lines
Abstract
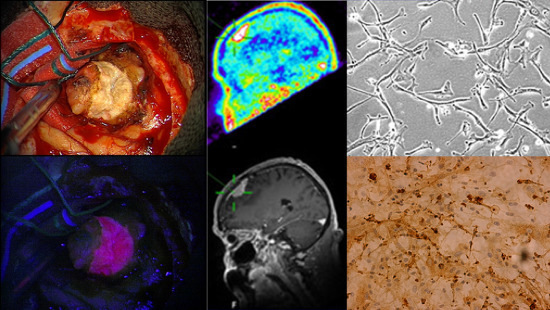
:1. Introduction
2. Results
2.1. Immunohistochemistry

2.2. Five-Aminolevulinic Acid (5-ALA) Photodynamic Therapy



3. Discussion
4. Experimental Section
4.1. Meningiomas
4.2. Cell Culture
4.3. Immunohistochemistry
4.4. Photodynamic Therapy (PDT)


4.5. WST-1 (Water Soluble Tetrazolium Salt) Cell Viability Assay
4.6. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Longstreth, W.T., Jr.; Dennis, L.K.; McGuire, V.M.; Drangsholt, M.T.; Koepsell, T.D. Epidemiology of intracranial meningioma. Cancer 1993, 72, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Rockhill, J.; Mrugala, M.; Chamberlain, M.C. Intracranial meningiomas: An overview of diagnosis and treatment. Neurosurg. Focus 2007, 23, E1. [Google Scholar] [CrossRef] [PubMed]
- Kuratsu, J.; Kochi, M.; Ushio, Y. Incidence and clinical features of asymptomatic meningiomas. J. Neurosurg. 2000, 92, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Mirimanoff, R.O.; Dosoretz, D.E.; Linggood, R.M.; Ojemann, R.G.; Martuza, R.L. Meningioma: Analysis of recurrence and progression following neurosurgical resection. J. Neurosurg. 1985, 62, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.L.; Perry, A.; Suman, V.J.; Meyer, F.B.; Scheithauer, B.W.; Lohse, C.M.; Shaw, E.G. Primarily resected meningiomas: Outcome and prognostic factors in 581 mayo clinic patients, 1978 through 1988. Mayo Clin. Proc. 1998, 73, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Ohgari, Y.; Nakayasu, Y.; Kitajima, S.; Sawamoto, M.; Mori, H.; Shimokawa, O.; Matsui, H.; Taketani, S. Mechanisms involved in δ-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: Relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem. Pharmacol. 2005, 71, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Whittle, I.R.; Smith, C.; Navoo, P.; Collie, D. Meningiomas. Lancet 2004, 363, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, M.; El Majdoub, F.; Hoevels, M.; Kocher, M.; Muller, R.P.; Steiger, H.J.; Sturm, V.; Maarouf, M. Stereotactic linac radiosurgery for incompletely resected or recurrent atypical and anaplastic meningiomas. Acta Neurochir. 2011, 153, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Lunsford, L.D.; Coffey, R.J.; Flickinger, J.C. Stereotactic radiosurgery of meningiomas. J. Neurosurg. 1991, 74, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Mathieu, D.; Lunsford, L.D.; Martin, J.J.; Madhok, R.; Niranjan, A.; Flickinger, J.C. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008, 62, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.L.; Pollock, B.E.; Foote, R.L.; Link, M.J.; Gorman, D.A.; Schomberg, P.J.; Leavitt, J.A. Meningioma radiosurgery: Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001, 49, 1029–1037. [Google Scholar] [PubMed]
- Hefti, M.; von Campe, G.; Moschopulos, M.; Siegner, A.; Looser, H.; Landolt, H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: A one-year experience at a single institutuion. Swiss Med. Wkly. 2008, 138, 180–185. [Google Scholar] [PubMed]
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J. Clin. Laser Med. Surg. 1996, 14, 289–304. [Google Scholar] [PubMed]
- Stummer, W.; Gotz, C.; Hassan, A.; Heimann, A.; Kempski, O. Kinetics of photofrin II in perifocal brain edema. Neurosurgery 1993, 33, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Hassan, A.; Kempski, O.; Goetz, C. Photodynamic therapy within edematous brain tissue: Considerations on sensitizer dose and time point of laser irradiation. J. Photochem. Photobiol. B 1996, 36, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Sun, C.H.; Tromberg, B.J.; Madsen, S.J. ALA- and ALA-ester-mediated photodynamic therapy of human glioma spheroids. J. Neurooncol. 2002, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olzowy, B.; Hundt, C.S.; Stocker, S.; Bise, K.; Reulen, H.J.; Stummer, W. Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J. Neurosurg. 2002, 97, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.W.; Tonn, J.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Hsiao, Y.Y.; Teng, L.J.; Chen, C.T.; Kao, M.C. Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg. Med. 1999, 24, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hebeda, K.M.; Saarnak, A.E.; Olivo, M.; Sterenborg, H.J.; Wolbers, J.G. 5-aminolevulinic acid induced endogenous porphyrin fluorescence in 9l and c6 brain tumours and in the normal rat brain. Acta Neurochir. 1998, 140, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Matsuo, T.; Hayashi, Y.; Suyama, K.; Nagata, I. Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: Technical case report. Neurosurgery 2008, 62, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Krammer, B.; Plaetzer, K. ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 2008, 7, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; Gong, J. 5-Aminolevulinate synthase and the first step of heme biosynthesis. J. Bioenerg. Biomembr. 1995, 27, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, Y.; Kuroiwa, T.; Miyatake, S.; Ichioka, T.; Miyashita, M.; Tanaka, H.; Tsuji, M. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J. Neurosurg. 2007, 106, 1070–1074. [Google Scholar]
- Hefti, M.; Holenstein, F.; Albert, I.; Looser, H.; Luginbuehl, V. Susceptibility to 5-aminolevulinic acid based photodynamic therapy in who i meningioma cells corresponds to ferrochelatase activity. Photochem. Photobiol. 2011, 87, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Fandino, J.; Fujioka, M.; Cordovi, S.; Muroi, C.; Landolt, H. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir. 2010, 152, 1711–1719. [Google Scholar] [CrossRef]
- Marks, P.V.; Furneaux, C.; Shivvakumar, R. An in vitro study of the effect of photodynamic therapy on human meningiomas. Br. J. Neurosurg. 1992, 6, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J.F.; Slotty, P.J. Meningioma surgery in the era of 5-aminolevulinic acid fluorescence-guided surgery. J. Neurosurg. 2014, 121, 766. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J.F.; Slotty, P.J.; El Khatib, M.; Giannakis, A.; Senger, B.; Steiger, H.J. Enhancing the effect of 5-aminolevulinic acid based photodynamic therapy in human meningioma cells. Photodiagn. Photodyn. Ther. 2014, 11, 1–6. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Slotty, P.J.; Kamp, M.A.; Schneiderhan, T.M.; Steiger, H.J.; El-Khatib, M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas—With special regard to high-grade tumors. Photodiagn. Photodyn. Ther. 2014, 11, 481–490. [Google Scholar] [CrossRef]
- Bekelis, K.; Valdes, P.A.; Erkmen, K.; Leblond, F.; Kim, A.; Wilson, B.C.; Harris, B.T.; Paulsen, K.D.; Roberts, D.W. Quantitative and qualitative 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in skull base meningiomas. Neurosurg. Focus 2011, 30, E8. [Google Scholar] [PubMed]
- Hefti, M. Comment concerning: Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas, Acta Neurochir doi 1o.1007/s00701-010-0708-4, intratumoral heterogeneity and fluorescence intensity in meningioma after 5-ALA pretreatment. Acta Neurochir. 2011, 153, 959–960. [Google Scholar]
- Pfisterer, W.K.; Hank, N.C.; Preul, M.C.; Hendricks, W.P.; Pueschel, J.; Coons, S.W.; Scheck, A.C. Diagnostic and prognostic significance of genetic regional heterogeneity in meningiomas. Neuro-Oncology 2004, 6, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sayagues, J.M.; Tabernero, M.D.; Maillo, A.; Espinosa, A.; Rasillo, A.; Diaz, P.; Ciudad, J.; Lopez, A.; Merino, M.; Goncalves, J.M.; et al. Intratumoral patterns of clonal evolution in meningiomas as defined by multicolor interphase fluorescence in situ hybridization (fish): Is there a relationship between histopathologically benign and atypical/anaplastic lesions? J. Mol. Diagn. 2004, 6, 316–325. [Google Scholar]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin ix, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 1992, 14, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Karashima, T.; Kamada, M.; Shuin, T.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J. Regulation of 5-aminolevulinic acid-mediated protoporphyrin IX accumulation in human urothelial carcinomas. Pathobiology 2009, 76, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.J.; Christodoulides, M.; Weller, R.O.; Heckels, J.E. Interactions of neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 2000, 36, 817–829. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Khatib, M.; Tepe, C.; Senger, B.; Dibué-Adjei, M.; Riemenschneider, M.J.; Stummer, W.; Steiger, H.J.; Cornelius, J.F. Aminolevulinic Acid-Mediated Photodynamic Therapy of Human Meningioma: An in Vitro Study on Primary Cell Lines. Int. J. Mol. Sci. 2015, 16, 9936-9948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16059936
El-Khatib M, Tepe C, Senger B, Dibué-Adjei M, Riemenschneider MJ, Stummer W, Steiger HJ, Cornelius JF. Aminolevulinic Acid-Mediated Photodynamic Therapy of Human Meningioma: An in Vitro Study on Primary Cell Lines. International Journal of Molecular Sciences. 2015; 16(5):9936-9948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16059936
Chicago/Turabian StyleEl-Khatib, Mustafa, Carolin Tepe, Brigitte Senger, Maxine Dibué-Adjei, Markus Johannes Riemenschneider, Walter Stummer, Hans Jakob Steiger, and Jan Frédérick Cornelius. 2015. "Aminolevulinic Acid-Mediated Photodynamic Therapy of Human Meningioma: An in Vitro Study on Primary Cell Lines" International Journal of Molecular Sciences 16, no. 5: 9936-9948. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms16059936