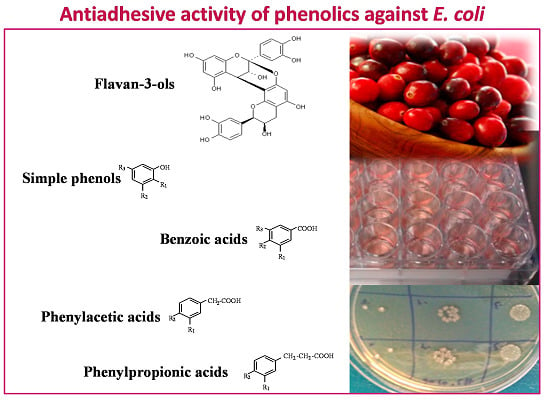
Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures
Abstract
:1. Introduction
2. Results

| Phenolic Compounds | C (µM) | ||
|---|---|---|---|
| 100 | 250 | 500 | |
| Flavan-3-ols | |||
| Procyanidin A2 | 7.63 ± 20.53 | −5.89 ± 10.42 | 51.3 ** ± 7.1 |
| Procyanidin B2 | 6.79 ± 22.15 | 10.0 ± 10.1 | −14.7 ± 20.9 |
| (−)-Epicatechin | −6.02 ± 21.95 | −1.21 ± 20.76 | −5.82 ± 23.28 |
| Simple Phenols | |||
| 1,2-Dihydroxybenzene (catechol/pyrocatechol) | 17.0 * ± 10.4 | 26.0 * ± 17.0 | 33.2 ** ± 11.7 |
| 1,3,5-Trihydroxybenzene (phloroglucinol) | −8.53 ± 10.55 | 17.6 ± 35.7 | −8.15 ± 29.41 |
| Benzoic Acids | |||
| Benzoic acid | 16.5 * ± 12.9 | 23.3 ** ± 14.0 | 32.2 ** ± 11.4 |
| 3-Hydroxybenzoic acid | 11.1 ± 30.4 | 17.0 * ± 9.1 | −9.7 ± 36.3 |
| 3,4-Dihydroxybenzoic acid (protocatechuic acid) | 25.5 * ± 10.2 | 24.0 ± 31.8 | 9.44 ± 17.09 |
| 4-Hydroxy-3-methoxybenzoic acid (vanillic acid) | 18.3 ** ± 2.3 | 24.9 ** ± 4.0 | 29.2 ** ± 2.2 |
| 3,4,5-Trihydroxybenzoic acid (gallic acid) | −3.72 ± 14.96 | 19.7 ± 43.7 | 40.6 ** ± 20.2 |
| Phenylacetic Acids | |||
| Phenylacetic acid | 33.5 * ± 28.7 | 39.0 ** ± 3.3 | 40.6 ** ± 10.1 |
| 3-Hydroxyphenylacetic acid | 15.0 ± 11.4 | 11.9 ± 21.8 | 19.4 ± 22.9 |
| 3,4-Dihydroxyphenylacetic acid | 23.6 * ± 13.6 | 32.5 * ± 22.2 | 37.0 ** ± 20.5 |
| Phenylpropionic Acids | |||
| 3-Phenylpropionic acid | −11.8 ± 27.2 | 14.7 ± 21.8 | 12.2 ± 13.6 |
| 3-(3-Hydroxyphenyl)-propionic acid | 10.2 ± 17.6 | 18.6 ± 25.4 | 30.5 * ± 27.4 |
| 3-(3,4-Dihydroxyphenyl)-propionic acid | 6.66 ± 10.63 | 1.19 ± 22.80 | 13.1 ± 15.4 |
| Phenolic Extracts | C (mg/L−1) | ||
|---|---|---|---|
| 200 | 500 | 1000 | |
| Cranberry extract | −11.9 ± 13.0 | −9.76 ± 8.65 | −13.8 ± 16.5 |
| Grape seed extract | 2.21 ± 9.01 | −16.4 ± 28.9 | −9.8 ± 15.8 |
3. Discussion
4. Experimental Section
4.1. Phenolic Compounds, Phenolic Extracts and Other Chemicals
4.2. Preparation of Phenolic Solutions
4.3. Escherichia coli Strain and Growing Conditions
4.4. Antibacterial Activity Assay
4.5. Cell Culture
4.6. Cytotoxicity Assay/Cell Viability
4.7. Adherence Assay
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.C.; Mobley, H.L. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ermel, G.; Georgeault, S.; Inisan, C.; Besnard, M. Inhibition of adhesion of uropathogenic Escherichia coli bacteria to uroepithelial cells by extracts from cranberry. J. Med. Food 2012, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Black, M.A.; Caron, L.; Camesano, T.A. Role of cranberry juice on molecular-scale surface characteristics and adhesion behavior of Escherichia coli. Biotechnol. Bioeng. 2006, 93, 297–305. [Google Scholar] [PubMed]
- Rafsanjany, N.; Lechtenberg, M.; Petereit, F.; Hensel, A. Antiadhesion as a functional concept for protection against uropathogenic Escherichia coli: In vitro studies with traditionally used plants with antiadhesive activity against uropathognic Escherichia coli. J. Ethnopharmacol. 2013, 145, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Papas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, I.; Katsargyris, A.; Theocharis, S.; Giaginis, C. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. Nutr. Res. 2013, 33, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD001321. [Google Scholar] [PubMed]
- Wang, C.H.; Fang, C.C.; Chen, N.C.; Liu, S.S.H.; Yu, P.H.; Wu, T.Y.; Chen, W.T.; Lee, C.C.; Chen, S.C. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: A systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Howell, A. Updated systematic review suggests that cranberry juice is not effective at preventing urinary tract infection. Evid. Based Nurs. 2013, 16, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, R.; Peng, N.; Moore, R.; Arabshahi, A.; Wyss, J.M.; Barnes, S.; Prasain, J.K. Determination of cranberry phenolic metabolites in rats by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6682–6688. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A. Absorption, metabolism, and excretion of (−)-epicatechin in humans: An evaluation of recent findings. Am. J. Clin. Nutr. 2013, 98, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Engemann, A.; Hübner, F.; Rzeppa, S.; Humpf, H.U. Intestinal metabolism of two a-type procyanidins using the pig cecum model: Detailed structure elucidation of unknown catabolites with fourier transform mass spectrometry (FTMS). J. Agric. Food Chem. 2012, 60, 749–757. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Valentova, K.; Stejskal, D.; Bednar, P.; Vostalova, J.; Cihalik, C.; Vecerova, R.; Koukalova, D.; Kolar, M.; Reichenbach, R.; Sknouril, L.; et al. Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: A pilot double-blind placebo-controlled trial. J. Agric. Food Chem. 2007, 55, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zuo, Y.; Vinson, J.A.; Deng, Y. Absorption and excretion of cranberry-derived phenolics in humans. Food Chem. 2012, 132, 1420–1428. [Google Scholar] [CrossRef]
- Di Martino, P.; Agniel, R.; David, K.; Templer, C.; Gaillard, J.L.; Denys, P.; Botto, H. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: A double-blind randomized placebo-controlled cross-over trial. World J. Urol. 2006, 24, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. BMC Infect. Dis. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, K.S.; Kim, Y.W.; Lee, W.H.; Choi, J.W. Protein array consisting of sol-gel bioactive platform for detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.P.; Bourg, G.; Combescure, C.; Botto, H.; Sotto, A. In vitro and in vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin. Microbiol. Infect. 2008, 14, 350–355. [Google Scholar] [PubMed]
- Stapleton, A.E.; Dziur, J.; Hooton, T.M.; Cox, M.E. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: A randomized controlled trial. Mayo Clin. Proc. 2012, 87, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dwivedi, M.; Mahdi, A.A.; Nagana Gowda, G.A.; Khetrapal, C.L.; Bhandari, M. Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidin. Urol. Res. 2012, 40, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Chou, M.Y.; Howell, A.; Wobbe, C.; Grady, R.; Stapleton, A.E. Cranberry products inhibit adherence of uropathogenic Escherichia coli to primary cultured bladder and vaginal epithelial cells. J. Urol. 2007, 177, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Bartolomé, B.; Martín-Álvarez, P.J.; Anderson, M.; Howell, A.; Monagas, M. Comprehensive assessment of the quality of commercial cranberry products. Phenolic characterization and in vitro bioactivity. J. Agric. Food Chem. 2012, 60, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Iswaldi, I.; Arraez-Roman, D.; Gomez-Caravaca, A.M.; Contreras Mdel, M.; Uberos, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Identification of polyphenols and their metabolites in human urine after cranberry-syrup consumption. Food Chem. Toxicol. 2013, 55, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Fumeaux, R.; Menozzi-Smarrito, C.; Stalmach, A.; Munari, C.; Kraehenbuehl, K.; Steiling, H.; Crozier, A.; Williamson, G.; Barron, D. First synthesis, characterization, and evidence for the presence of hydroxycinnamic acid sulfate and glucuronide conjugates in human biological fluids as a result of coffee consumption. Org. Biomol. Chem. 2010, 8, 5199–5211. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-Cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia Coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Brinton, C.C.; Fusco, D.C. Vaccine composition for immunization against urinary tract infection caused by E. coli. U.S. Patent 4,725,435, 16 February 1988. [Google Scholar]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, D.W.; Pascal, K.E.; Libby, E.K.; Mordechai, E.; Adelson, M.E.; Trama, J.P. Uropathogenic Escherichia coli dominantly suppress the innate immune response of bladder epithelial cells by a lipopolysaccharide- and toll-Like receptor 4-independent pathway. Microbes Infect. 2008, 10, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and citotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González de Llano, D.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martínlvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures. Int. J. Mol. Sci. 2015, 16, 12119-12130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160612119
González de Llano D, Esteban-Fernández A, Sánchez-Patán F, Martínlvarez PJ, Moreno-Arribas MV, Bartolomé B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures. International Journal of Molecular Sciences. 2015; 16(6):12119-12130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160612119
Chicago/Turabian StyleGonzález de Llano, Dolores, Adelaida Esteban-Fernández, Fernando Sánchez-Patán, Pedro J. Martínlvarez, Mª Victoria Moreno-Arribas, and Begoña Bartolomé. 2015. "Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures" International Journal of Molecular Sciences 16, no. 6: 12119-12130. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160612119