Behavioral Deficits Are Accompanied by Immunological and Neurochemical Changes in a Mouse Model for Neuropsychiatric Lupus (NP-SLE)
Abstract
:1. Introduction

2. Results
2.1. MRL/MpJ-Faslpr (MRL/lpr) Mice Exhibit Depression-Like Behavior without Anxiety-Like Behavior

2.2. MRL/lpr Mice Displayed Cognitive Deficits in Visuospatial Memory but Not in Object Recognition Memory

2.3. MRL/lpr Mice Had Higher Levels of Several Plasma Biomarkers Related to Inflammation


2.4. MRL/lpr Mice Exhibit Altered Tryptophan and Kynurenine Metabolism


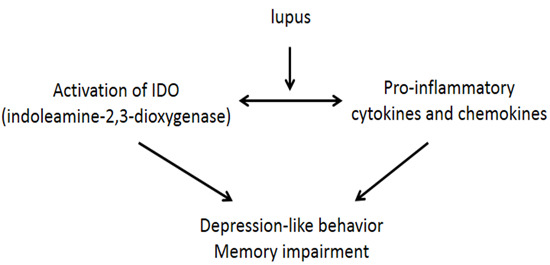
3. Discussion
4. Materials and Methods
4.1. Animals and Materials
4.2. Behavioral Tests
4.3. Sample Collection
4.4. Quantification of Chemokines and Cytokines
4.5. Liquid Chromatography-Tandem Mass Spectrometry Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Ainiala, H.; Loukkola, J.; Peltola, J.; Korpela, M.; Hietaharju, A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001, 57, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Steup-Beekman, G.M.; Zirkzee, E.J.; Cohen, D.; Gahrmann, B.M.; Emmer, B.J.; Steens, S.C.; Bollen, E.L.; van Buchem, M.A.; Huizinga, T.W. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: Epidemiology and radiology pointing to an immune-mediated cause. Ann. Rheum. Dis. 2013, 72, ii76–ii79. [Google Scholar] [CrossRef] [PubMed]
- Gulinello, M.; Wen, J.; Putterman, C. Neuropsychiatric Symptoms in Lupus. Psychiatr. Ann. 2012, 42, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Putterman, C.; Naparstek, Y. Neuropsychiatric involvement in systemic lupus erythematosus. Isr. J. Med. Sci. 1992, 28, 458–460. [Google Scholar] [PubMed]
- Stock, A.D.; Wen, J.; Putterman, C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front. Immunol. 2013, 4, 484. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xia, Y.; Stock, A.; Michaelson, J.S.; Burkly, L.C.; Gulinello, M.; Putterman, C. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J. Autoimmun. 2013, 43, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Carbotte, R.M.; Denburg, S.D.; Denburg, J.A. Cognitive dysfunction in systemic lupus erythematosus is independent of active disease. J. Rheumatol. 1995, 22, 863–867. [Google Scholar] [PubMed]
- Kasitanon, N.; Louthrenoo, W.; Piyasirisilp, S.; Sukitawu, W.; Wichainun, R. Neuropsychiatric manifestations in Thai patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2002, 61, 96. [Google Scholar]
- Gao, H.X.; Campbell, S.R.; Cui, M.H.; Zong, P.; Hee-Hwang, J.; Gulinello, M.; Putterman, C. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J. Neuroimmunol. 2009, 207, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ballok, D.A.; Ma, X.; Denburg, J.A.; Arsenault, L.; Sakic, B. Ibuprofen fails to prevent brain pathology in a model of neuropsychiatric lupus. J. Rheumatol. 2006, 33, 2199–2213. [Google Scholar] [PubMed]
- Gao, H.X.; Sanders, E.; Tieng, A.T.; Putterman, C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J. Neuroimmunol. 2010, 229, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Gulinello, M.; Putterman, C. The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Szechtman, H.; Sakic, B.; Denburg, J.A. Behaviour of MRL mice: An animal model of disturbed behavior in systemic autoimmune disease. Lupus 1997, 6, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Sakic, B.; Hoffman, S.A. Circulating brain-reactive autoantibodies and behavioral deficits in the MRL model of CNS lupus. J. Neuroimmunol. 2010, 218, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Stock, A.; Wang, H.W.; Shlomchik, M.; Gulinello, M.; Putterman, C. Hold the rituximab: Neuropsychiatric disease in murine lupus is not B-cell dependent. Arthritis Rheum. 2013, 65, S244. [Google Scholar]
- Wen, J.; Stock, A.; Wang, H.W.; Shlomchik, M.; Gulinello, M.; Putterman, C. Neuropsychiatric lupus is substantially unaffected by B-cell deficiency. Arthritis Rheum. 2014, 66, S855. [Google Scholar]
- Alleva, D.G.; Kaser, S.B.; Beller, D.I. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J. Immunol. 1997, 159, 5610–5619. [Google Scholar] [PubMed]
- Balomenos, D.; Rumold, R.; Theofilopoulos, A.N. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J. Clin. Investig. 1998, 101, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Boswell, J.M.; Yui, M.A.; Endres, S.; Burt, D.W.; Kelley, V.E. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J. Immunol. 1988, 141, 118–124. [Google Scholar] [PubMed]
- Braun, D.; Geraldes, P.; Demengeot, J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J. Autoimmun. 2003, 20, 15–25. [Google Scholar] [CrossRef]
- Esfandiari, E.; McInnes, I.B.; Lindop, G.; Huang, F.P.; Field, M.; Komai-Koma, M.; Wei, X.; Liew, F.Y. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J. Immunol. 2001, 167, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.S.; Wang, Z.; Levine, J.S. Cytokine dysregulation induced by apoptotic cells is a shared characteristic of murine lupus. J. Immunol. 2000, 165, 4190–4201. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflamm. 2014, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Metz, R.; Muller, A.J.; Merlo, L.M.; Mandik-Nayak, L. IDO2 in immunomodulation and autoimmune disease. Front. Immunol. 2014, 5, 585. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Appenzeller, S. The importance of cytokines and autoantibodies in depression. Autoimmun. Rev. 2015, 14, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, M.; Hasan, T.; Raitala, A.; Oja, S.S.; Yli-Kerttula, U.; Korpela, M.; Hurme, M. Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin. Exp. Immunol. 2007, 150, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Sepp, N.; Kowald, E.; Kind, S.; Schmuth, M.; Fuchs, D. Degradation of tryptophan in patients with systemic lupus erythematosus. Adv. Exp. Med. Biol. 1999, 467, 571–577. [Google Scholar] [PubMed]
- Widner, B.; Sepp, N.; Kowald, E.; Ortner, U.; Wirleitner, B.; Fritsch, P.; Baier-Bitterlich, G.; Fuchs, D. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 2000, 201, 621–630. [Google Scholar] [CrossRef]
- Capuron, L.; Dantzer, R. Cytokines and depression: The need for a new paradigm. Brain Behav. Immun. 2003, 17, S119–S124. [Google Scholar] [CrossRef]
- Merlo, L.M.; Pigott, E.; DuHadaway, J.B.; Grabler, S.; Metz, R.; Prendergast, G.C.; Mandik-Nayak, L. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J. Immunol. 2014, 192, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Frenois, F.; Moreau, M.; O’Connor, J.; Lawson, M.; Micon, C.; Lestage, J.; Kelley, K.W.; Dantzer, R.; Castanon, N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007, 32, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Clarke, G.; O’Neill, A.; Groeger, J.A.; Quigley, E.M.; Shanahan, F.; Cryan, J.F.; Dinan, T.G. Cognitive performance in irritable bowel syndrome: Evidence of a stress-related impairment in visuospatial memory. Psychol. Med. 2014, 44, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Andre, C.; Wang, Y.; Lawson, M.A.; Szegedi, S.S.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009, 29, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Briley, E.M.; Szegedi, S.S.; Lestage, J.; Castanon, N.; Herkenham, M.; Dantzer, R.; Kelley, K.W. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J. Immunol. 2009, 182, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Too, L.K.; Mitchell, A.J.; Yau, B.; Ball, H.J.; McGregor, I.S.; Hunt, N.H. Interleukin-18 deficiency and its long-term behavioural and cognitive impacts in a murine model of pneumococcal meningitis. Behav. Brain Res. 2014, 263, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Dantzer, R.; Kelley, K.W.; Lawson, M.A.; Woolwine, B.J.; Vogt, G.; Spivey, J.R.; Saito, K.; Miller, A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Mol. Psychiatry 2010, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Filippini, P.; del Papa, N.; Sambataro, D.; del Bufalo, A.; Locatelli, F.; Rutella, S. Emerging concepts on inhibitors of indoleamine 2,3-dioxygenase in rheumatic diseases. Curr. Med. Chem. 2012, 19, 5381–5393. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M.; Bondy, B.; Baghai, T.C.; Eser, D.; Nothdurfter, C.; Schule, C.; Zill, P.; Muller, N.; Rupprecht, R.; Schwarz, M.J. Tryptophan metabolism and immunogenetics in major depression: A role for interferon-γ gene. Brain Behav. Immun. 2013, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Chrusciel, M. Changes resembling lupus erythematosus after prolonged treatment with Ro-4-4602, a potent inhibitor of 5-HTP-carboxyliase, in white rats. Eur. J. Pharmacol. 1969, 8, 192–199. [Google Scholar] [CrossRef]
- Hoyo-Becerra, C.; Schlaak, J.F.; Hermann, D.M. Insights from interferon-α-related depression for the pathogenesis of depression associated with inflammation. Brain Behav. Immun. 2014, 42, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.Y.; Tang, A.G.; Ren, Y.P.; Zhou, Q.X.; Luo, X.B. Simultaneous determination of serum tryptophan metabolites in patients with systemic lupus erythematosus by high performance liquid chromatography with fluorescence detection. Clin. Chem. Lab. Med. 2010, 48, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. Pharmacology and regional variations of quinolinic acid-evoked excitations in the rat central nervous system. J. Pharmacol. Exp. Ther. 1983, 226, 551–557. [Google Scholar] [PubMed]
- Hughes, P.E.; Alexi, T.; Yoshida, T.; Schreiber, S.S.; Knusel, B. Excitotoxic lesion of rat brain with quinolinic acid induces expression of p53 messenger RNA and protein and p53-inducible genes Bax and Gadd-45 in brain areas showing DNA fragmentation. Neuroscience 1996, 74, 1143–1160. [Google Scholar] [CrossRef]
- Moresco, R.M.; Lavazza, T.; Belloli, S.; Lecchi, M.; Pezzola, A.; Todde, S.; Matarrese, M.; Carpinelli, A.; Turolla, E.; Zimarino, V.; et al. Quinolinic acid induced neurodegeneration in the striatum: A combined in vivo and in vitro analysis of receptor changes and microglia activation. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Velloso, N.A.; Dalmolin, G.D.; Gomes, G.M.; Rubin, M.A.; Canas, P.M.; Cunha, R.A.; Mello, C.F. Spermine improves recognition memory deficit in a rodent model of Huntington’s disease. Neurobiol. Learn. Mem. 2009, 92, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Walker, A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. 2014, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Shear, D.A.; Dong, J.; Haik-Creguer, K.L.; Bazzett, T.J.; Albin, R.L.; Dunbar, G.L. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp. Neurol. 1998, 150, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; Meyer zu Schwabedissen, L.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 2011, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.W.; Perkins, M.N. Quinolinic acid: A potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981, 72, 411–412. [Google Scholar] [CrossRef]
- Schwarcz, R.; Kohler, C. Differential vulnerability of central neurons of the rat to quinolinic acid. Neurosci. Lett. 1983, 38, 85–90. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.Q.; Potter, M.C.; Elmer, G.I.; Pellicciari, R.; Schwarcz, R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 2011, 36, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Sakic, B.; Denburg, J.A.; Denburg, S.D.; Szechtman, H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: A curve-shift study. Brain Res. Bull. 1996, 41, 305–311. [Google Scholar] [CrossRef]
- Efthimiou, P.; Blanco, M. Pathogenesis of neuropsychiatric systemic lupus erythematosus and potential biomarkers. Mod. Rheumatol. 2009, 19, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Funauchi, M.; Sugishima, H.; Minoda, M.; Horiuchi, A. Serum level of interferon-γ in autoimmune diseases. Tohoku J. Exp. Med. 1991, 164, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kwant, A.; Sakic, B. Behavioral effects of infection with interferon-γ adenovector. Behav. Brain Res. 2004, 151, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Schrott, L.M.; Crnic, L.S. Attenuation of behavioral abnormalities in autoimmune mice by chronic soluble interferon-γ receptor treatment. Brain Behav. Immun. 1998, 12, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Svenungsson, E.; Andersson, M.; Brundin, L.; van Vollenhoven, R.; Khademi, M.; Tarkowski, A.; Greitz, D.; Dahlstrom, M.; Lundberg, I.; Klareskog, L.; et al. Increased levels of proinflammatory cytokines and nitric oxide metabolites in neuropsychiatric lupus erythematosus. Ann. Rheum. Dis. 2001, 60, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Blenman, K.R.; Duan, B.; Xu, Z.; Wan, S.; Atkinson, M.A.; Flotte, T.R.; Croker, B.P.; Morel, L. IL-10 regulation of lupus in the NZM2410 murine model. Lab. Investig. 2006, 86, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.Y.; Chung, J.W.; Kim, H.A.; Yun, J.M.; Jeon, J.Y.; Ye, Y.M.; Kim, S.H.; Park, H.S.; Suh, C.H. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J. Clin. Immunol. 2007, 27, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Rood, M.J.; Keijsers, V.; van der Linden, M.W.; Tong, T.Q.; Borggreve, S.E.; Verweij, C.L.; Breedveld, F.C.; Huizinga, T.W. Neuropsychiatric systemic lupus erythematosus is associated with imbalance in interleukin 10 promoter haplotypes. Ann. Rheum. Dis. 1999, 58, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Li, J.L.; Wang, D.G.; Zhou, D. Targeting IL-10 in auto-immune diseases. Cell Biochem. Biophys. 2014, 70, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bahtiyar, G.; Zhang, N.; Liu, L.; Zhu, P.; Robert, M.E.; McNiff, J.; Madaio, M.P.; Craft, J. IL-10 regulates murine lupus. J. Immunol. 2002, 169, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Talaat, R.M.; Mohamed, S.F.; Bassyouni, I.H.; Raouf, A.A. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 2015, 72, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Elia, G. Interferon-γ-Inducible chemokines in Systemic Lupus Erythematosus. Clin. Ther. 2015, 166, e41–e46. [Google Scholar]
- Kong, K.O.; Tan, A.W.; Thong, B.Y.; Lian, T.Y.; Cheng, Y.K.; Teh, C.L.; Koh, E.T.; Chng, H.H.; Law, W.G.; Lau, T.C.; et al. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin. Exp. Immunol. 2009, 156, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Zhu, C.Q.; Qian, J.; Chen, X.X.; Ye, S.; Gu, Y.Y. Intrathecal cytokine and chemokine profiling in neuropsychiatric lupus or lupus complicated with central nervous system infection. Lupus 2010, 19, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Iikuni, N.; Kamitsuji, S.; Yoshio, T.; Minota, S.; Kamatani, N. IP-10/MCP-1 ratio in CSF is an useful diagnostic marker of neuropsychiatric lupus patients. Rheumatology 2006, 45, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Santer, D.M.; Yoshio, T.; Minota, S.; Moller, T.; Elkon, K.B. Potent induction of IFN-α and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J. Immunol. 2009, 182, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.W.; Petri, M.; Batliwalla, F.M.; Koeuth, T.; Wilson, J.; Slattery, C.; Panoskaltsis-Mortari, A.; Gregersen, P.K.; Behrens, T.W.; Baechler, E.C. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 2009, 60, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.; Hrycek, A. Pentraxin 3 as a biomarker of local inflammatory response to vascular injury in systemic lupus erythematosus. Autoimmunity 2014, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iikuni, N.; Okamoto, H.; Yoshio, T.; Sato, E.; Kamitsuji, S.; Iwamoto, T.; Momohara, S.; Taniguchi, A.; Yamanaka, H.; Minota, S.; et al. Raised monocyte chemotactic protein-1 (MCP-1)/CCL2 in cerebrospinal fluid of patients with neuropsychiatric lupus. Ann. Rheum. Dis. 2006, 65, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Wanchu, A.; Bhatnagar, A. Interaction between oxidative stress and chemokines: Possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology 2011, 216, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Wu, T.; Xie, C.; Vanarsa, K.; Han, J.; Mahajan, T.; Oei, H.B.; Ahn, C.; Zhou, X.J.; Putterman, C.; et al. Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res. Ther. 2012, 14, R164. [Google Scholar]
- Zameer, A.; Hoffman, S.A. Increased ICAM-1 and VCAM-1 expression in the brains of autoimmune mice. J. Neuroimmunol. 2003, 142, 67–74. [Google Scholar] [CrossRef]
- Pavon, E.J.; Munoz, P.; Lario, A.; Longobardo, V.; Carrascal, M.; Abian, J.; Martin, A.B.; Arias, S.A.; Callejas-Rubio, J.L.; Sola, R.; et al. Proteomic analysis of plasma from patients with systemic lupus erythematosus: Increased presence of haptoglobin α2 polypeptide chains over the α1 isoforms. Proteomics 2006, 6, S282–S292. [Google Scholar]
- Finklestein, S.P.; Fanning, P.J.; Caday, C.G.; Powell, P.P.; Foster, J.; Clifford, E.M.; Klagsbrun, M. Increased levels of basic fibroblast growth factor (bFGF) following focal brain injury. Restor. Neurol. Neurosci. 1990, 1, 387–394. [Google Scholar]
- Gospodarowicz, D.; Neufeld, G.; Schweigerer, L. Fibroblast growth factor: Structural and biological properties. J. Cell. Physiol. 1987, 5, 15–26. [Google Scholar]
- Finklestein, S.P.; Apostolides, P.J.; Caday, C.G.; Prosser, J.; Philips, M.F.; Klagsbrun, M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res. 1988, 460, 253–259. [Google Scholar] [CrossRef]
- House, S.L.; Bolte, C.; Zhou, M.; Doetschman, T.; Klevitsky, R.; Newman, G.; Schultz Jel, J. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation 2003, 108, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Schweigerer, L.; Neufeld, G.; Friedman, J.; Abraham, J.A.; Fiddes, J.C.; Gospodarowicz, D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.M.; Borden, E.C.; Sondel, P.M.; Byrne, G.I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J. Immunol. 1987, 139, 2414–2418. [Google Scholar]
- Yasui, H.; Takai, K.; Yoshida, R.; Hayaishi, O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: Its possible occurrence in cancer patients. Proc. Natl. Acad. Sci. USA 1986, 83, 6622–6626. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar]
- Munn, D.H.; Sharma, M.D.; Lee, J.R.; Jhaver, K.G.; Johnson, T.S.; Keskin, D.B.; Marshall, B.; Chandler, P.; Antonia, S.J.; Burgess, R.; et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002, 297, 1867–1870. [Google Scholar]
- Yanagawa, Y.; Iwabuchi, K.; Onoe, K. Co-operative action of interleukin-10 and interferon-γ to regulate dendritic cell functions. Immunology 2009, 127, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Odemuyiwa, S.O.; Ghahary, A.; Li, Y.; Puttagunta, L.; Lee, J.E.; Musat-Marcu, S.; Ghahary, A.; Moqbel, R. Cutting edge: Human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J. Immunol. 2004, 173, 5909–5913. [Google Scholar] [CrossRef]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; et al. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar]
- Van Wetering, S.; van den Berk, N.; van Buul, J.D.; Mul, F.P.; Lommerse, I.; Mous, R.; ten Klooster, J.P.; Zwaginga, J.J.; Hordijk, P.L. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 2003, 285, C343–C352. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 2006, 26, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; She, R.; Huang, Y.; Fu, Z.F. Expression of neuronal CXCL10 induced by rabies virus infection initiates infiltration of inflammatory cells, production of chemokines and cytokines, and enhancement of blood-brain barrier permeability. J. Virol. 2015, 89, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Brew, B.J.; Letendre, S.L. Biomarkers of HIV related central nervous system disease. Int. Rev. Psychiatry 2008, 20, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Westin, K.; Buchhave, P.; Nielsen, H.; Minthon, L.; Janciauskiene, S.; Hansson, O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS ONE 2012, 7, e30525. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, A.; Meli, E.; Moroni, F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J. Neurochem. 2001, 77, 1310–1318. [Google Scholar] [CrossRef]
- Eastman, C.L.; Guilarte, T.R. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem. Res. 1990, 15, 1101–1107. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Plangar, I.; Majlath, Z.; Vecsei, L. Kynurenines in cognitive functions: Their possible role in depression. Neuropsychopharmacol. Hung. 2012, 14, 239–244. [Google Scholar]
- Capuron, L.; Neurauter, G.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Fuchs, D.; Miller, A.H. Interferon-α-induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biol. Psychiatry 2003, 54, 906–914. [Google Scholar] [CrossRef]
- Vogelgesang, S.A.; Heyes, M.P.; West, S.G.; Salazar, A.M.; Sfikakis, P.P.; Lipnick, R.N.; Klipple, G.L.; Tsokos, G.C. Quinolinic acid in patients with systemic lupus erythematosus and neuropsychiatric manifestations. J. Rheumatol. 1996, 23, 850–855. [Google Scholar]
- Ennaceur, A.; Meliani, K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav. Brain Res. 1992, 51, 83–92. [Google Scholar] [CrossRef]
- Aggleton, J.P.; Albasser, M.M.; Aggleton, D.J.; Poirier, G.L.; Pearce, J.M. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav. Neurosci. 2010, 124, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Albasser, M.M.; Davies, M.; Futter, J.E.; Aggleton, J.P. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav. Neurosci. 2009, 123, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Jopson, T.; Tweedie, D.; Arellano, C.; Luo, W.; Greig, N.H.; Rosi, S. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflamm. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Gokhan, S.; Gulinello, M.; Branch, C.A.; Patil, M.; Basu, R.; Stoddart, C.; Mehler, M.F.; Stanley, E.R. Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP). Neurobiol. Dis. 2015, 74, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.D.; Vijayanathan, V.; Ali, N.F.; Wagshul, M.E.; Tanenbaum, E.J.; Price, J.; Dalal, V.; Gulinello, M.E. Memantine protects rats treated with intrathecal methotrexate from developing spatial memory deficits. Clin. Cancer Res. 2013, 19, 4446–4454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vijayanathan, V.; Gulinello, M.; Cole, P.D. Intrathecal methotrexate induces focal cognitive deficits and increases cerebrospinal fluid homocysteine. Pharmacol. Biochem. Behav. 2010, 95, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vijayanathan, V.; Gulinello, M.E.; Cole, P.D. Systemic methotrexate induces spatial memory deficits and depletes cerebrospinal fluid folate in rats. Pharmacol. Biochem. Behav. 2010, 94, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Vijayanathan, V.; Gulinello, M.; Ali, N.; Cole, P.D. Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist. Behav. Brain Res. 2011, 225, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Quirion, R.; Meaney, M.J. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology 1989, 97, 277–279. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Porsolt, R.D. Animal model of depression. Biomedicine 1979, 30, 139–140. [Google Scholar]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Eskelund, A.R.; Zhou, H.; Budac, D.P.; Sánchez, C.; Gulinello, M. Behavioral Deficits Are Accompanied by Immunological and Neurochemical Changes in a Mouse Model for Neuropsychiatric Lupus (NP-SLE). Int. J. Mol. Sci. 2015, 16, 15150-15171. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160715150
Li Y, Eskelund AR, Zhou H, Budac DP, Sánchez C, Gulinello M. Behavioral Deficits Are Accompanied by Immunological and Neurochemical Changes in a Mouse Model for Neuropsychiatric Lupus (NP-SLE). International Journal of Molecular Sciences. 2015; 16(7):15150-15171. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160715150
Chicago/Turabian StyleLi, Yan, Amanda R. Eskelund, Hua Zhou, David P. Budac, Connie Sánchez, and Maria Gulinello. 2015. "Behavioral Deficits Are Accompanied by Immunological and Neurochemical Changes in a Mouse Model for Neuropsychiatric Lupus (NP-SLE)" International Journal of Molecular Sciences 16, no. 7: 15150-15171. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160715150