Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration
Abstract
:1. Introduction
2. Angiogenesis-Related Biomarkers of Placental Dysfunction in Physiological and Pathological Conditions
Clinical Keys for Interpreting Angiogenesis-Related Biomarkers in Pregnancy: The sFlt-1/PlGF Ratio

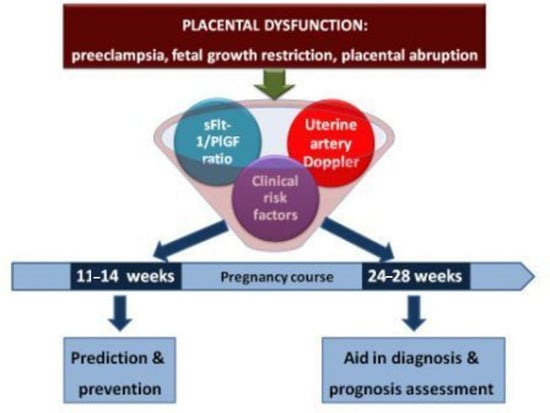
3. From Bench to Bedside: Rationale for the Clinical Implementation of the sFlt-1/PLGF Ratio
| Proposed Application | Comments |
|---|---|
| Prediction of PD and stratification of care | →First trimester (11–14 weeks): for the selection of women potentially benefit from the use of preventive measures such as low dose aspirin (only PlGF); →Second and third trimester: for the precise stratification of patients that should receive intensive following-up in a specialized center. |
| Early diagnosis of PD | sFlt-1/PlGF ratio increases significantly 5 to 8 weeks before the appearance of clinical manifestations. |
| Differential diagnosis | →Uncertain cases; →Atypical cases. |
| Management of PE | Categorize those cases susceptible to receive an expectant management. |
| Cost-efficiency | Avoidance of other diagnostic tests or reducing admissions for false PD suspicion. |
| Treatment | Development of new treatments based on the angiogenic balance regulation and monitorization of its efficacy (especially sFlt-1). |
3.1. First-Trimester Prediction and Prevention of Placental Dysfunction-Related Disorders
| Maternal History | Physical Examination | Ultrasound Findings | Serum Biomarkers | |
|---|---|---|---|---|
| High-Risk Factors | Moderate-Risk Factors | |||
| Previous PD related disorder † | Maternal age | Mean arterial BP | UA-mPI | PlGF |
| Chronic HT | Parity | Weight | PAPP-A | |
| Chronic kidney disease | Familiar history of PD related disorder † | Height | ||
| Diabetes Mellitus | Ethnicity | |||
| Trombophilia, SLE | Method of conception | |||
3.2. Second Trimester (24–28 Weeks): Stratification of Care in Asymptomatic Patients
3.3. Aid in Early Diagnosis in Symptomatic Patients
3.4. Aid in Management
4. Proposed Algorithm for the Clinical Integration of the sFlt-1/PLGF Ratio throughout Pregnancy


- sFlt-1/PlGF ratio under the “rule out” cut-off point (<38): there is a low risk of developing any early-onset PD, and women can undergo a regular follow-up, tailored to their pregnancy characteristics.
- sFlt-1/PlGF ratio in an intermediate range (38–85): these women are highly likely to develop clinical manifestations of PD within four weeks. A new biomarkers’ determination is needed two weeks later, as this is the lapse considered to be safe of pregnancy complications when sFlt-1/PlGF ratio values are elevated under 85.
- sFlt-1/PlGF ratio upper than the “rule in” cut-off point (>85), or symptomatic PD: those patients should be considered as having a PD, and managed as current guidelines, adding regular reappraisals of the sFlt-1/PlGF ratio every 48–96 h for the support of clinical management (Figure 2). If clinical diagnosis of a PD-related disorder has not been reached, it should be actively pursued.
5. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Friedman, A.M.; Cleary, K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014, 38, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, H.; Thadhani, R.; Benzing, T.; Karumanchi, S.A.; Stepan, H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin. Chem. 2012, 58, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Menzies, J.; Magee, L.A.; Macnab, Y.C.; Ansermino, J.M.; Li, J.; Douglas, M.J.; Gruslin, A.; Kyle, P.; Lee, S.K.; Moore, M.P.; et al. Current CHS and NHBPEP criteria for severe preeclampsia do not uniformly predict adverse maternal or perinatal outcomes. Hypertens. Pregnancy 2007, 26, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.R. Abruptio placentae and disseminated intravascular coagulopathy. Semin. Perinatol. 2009, 33, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M. Pathophysiology of ischemic placental disease. Semin. Perinatol. 2014, 38, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endotelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sircar, M.; Thadhani, R.; Karumanchi, S.A. Pathogenesis of preeclampsia. Curr. Opin. Nephrol. Hypertens. 2015, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.; Tong, S.; Palmer, K.R. Is fetal growth restriction associated with a more severe maternal phenotype in the setting of early onset pre-eclampsia? A retrospective study. PLoS ONE 2011, 6, e26937. [Google Scholar] [CrossRef] [PubMed]
- Signore, C.; Mills, J.L.; Qian, C.; Yu, K.; Lam, C.; Epstein, F.H.; Karumanchi, S.A.; Levine, R.J. Circulating angiogenic factors and placental abruption. Obstet. Gynecol. 2006, 108, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arriaga, P.I.; Herraiz, I.; López-Jiménez, E.A.; Escribano, D.; Denk, B.; Galindo, A. Uterine artery Doppler and sFlt-1/PlGF ratio: Prognostic value in early-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2014, 43, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Romero, R.; Korzeniewski, S.J.; Cortez, J.M.; Pappas, A.; Tarca, A.L.; Chaemsaithong, P.; Dong, Z.; Yeo, L.; Hassan, S.S. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: A prospective study. J. Matern. Fetal Neonatal Med. 2014, 27, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Catov, J.M. Preeclampsia more than 1 disease or is it? Hypertension 2008, 51, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Dröge, L.A.; Gómez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the soluble fms-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by Fetal Growth Restriction. Obstet. Gynecol. 2014, 124, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Schnettler, W.T.; Powe, C.; Wenger, J.; Salahuddin, S.; Cerdeira, A.S.; Verlohren, S.; Perschel, F.H.; Arany, Z.; Lim, K.H.; et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens. Pregnancy 2013, 32, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J. Abnormal fetal-maternal interactions: An evolutionary value? Obstet. Gynecol. 2012, 120, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Syngelaki, A.; Akolekar, R.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 2015, 213, 62–e1. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L.; Staff, A.C. IFPA Senior Award Lecture: Making sense of pre-eclampsia—Two placental causes of preeclampsia? Placenta 2014, 35 (Suppl. 102), 20–25. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J. Uteroplacental ischemia in early- and late-onset pre-eclampsia: A role for the fetus? Ultrasound Obstet. Gynecol. 2012, 40, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. A randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet 1994, 343, 619–629. [Google Scholar]
- Caritis, S.; Sibai, B.; Hauth, J.; Lindheimer, M.D.; Klebanoff, M.; Thom, E.; van Dorsten, P.; Landon, M.; Paul, R.; Miodovnik, M.; et al. Low-dose aspirin to prevent preeclampsia in women at high risk. N. Engl. J. Med. 1998, 338, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.K.; Papageorghiou, A.T.; Parra, M.; Palma Dias, R.; Nicolaides, K.H. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet. Gynecol. 2003, 22, 233–239. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health. Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy. Clinical Guideline; RCOG Press: London, UK, August 2010. [Google Scholar]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 2012, 122, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Mutter, W.P.; Wolf, M.; Levine, R.J.; Taylor, R.N.; Sukhatme, V.P.; Ecker, J.; Karumanchi, S.A. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J. Clin. Endocrinol. Metab. 2004, 89, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.U.; Bersinger, N.A.; Mohaupt, M.G.; Raio, L.; Gerber, S.; Surbek, D.V. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am. J. Obstet. Gynecol. 2008, 199, 266.e1–266.e6. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Syngelaki, A.; Sarquis, R.; Zvanca, M.; Nicolaides, K.H. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat. Diagn. 2011, 31, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Crispi, F.; Scazzocchio, E.; Mercade, I.; Meler, E.; Figueras, F.; Gratacos, E. First-trimester screening for early and late small-for-gestational-age neonates using maternal serum biochemistry, blood pressure and uterine artery Doppler. Ultrasound Obstet. Gynecol. 2014, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Magder, L.S.; Blitzer, M.G.; Baschat, A.A. First-trimester prediction of pre-eclampsia: External validity of algorithms in a prospectively enrolled cohort. Ultrasound Obstet. Gynecol. 2014, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, A.; Meiri, H.; Gonen, R. Economic assessment of screening for pre-eclampsia. Prenat. Diagn. 2012, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Abou, E.l.; Hassan, M.; Diamandis, E.P.; Karumanchi, S.A.; Shennan, A.H.; Taylor, R.N. Preeclampsia: An old disease with new tools for better diagnosis and risk management. Clin. Chem. 2015, 61, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arriaga, P.I.; Herraiz, I.; López-Jiménez, E.A.; Gómez-Montes, E.; Denk, B.; Galindo, A. Uterine artery Doppler and sFlt-1/PlGF ratio: Usefulness in diagnosis of pre-eclampsia. Ultrasound Obstet. Gynecol. 2013, 41, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Sagol, S.; Ozkinay, E.; Oztekin, K.; Ozdemir, N. The comparison of uterine artery Doppler velocimetry with the histopathology of the placental bed. Aust. N. Zeal. J. Obstet. Gnaecol. 1999, 39, 324–329. [Google Scholar] [CrossRef]
- Sciscione, A.C.; Hayes, E.J. Uterine artery Doppler flow studies in obstetric practice. Am. J. Obstet. Gynecol. 2009, 201, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Bindra, R.; Curcio, P.; Cicero, S.; Nicolaides, K.H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet. Gynecol. 2001, 18, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, J.S.; Morris, R.K.; ter Riet, G.; Mol, B.W.; van der Post, J.A.; Coomarasamy, A.; Zwinderman, A.H.; Robson, S.C.; Bindels, P.J.; Kleijnen, J.; et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: A aystematic review and bivariable meta-analysis. CMAJ 2008, 178, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E.; Khan, K.S.; Aquilina, J.; Thangaratinam, S. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Ghi, T.; Contro, E.; Youssef, A.; Giorgetta, F.; Farina, A.; Pilu, G.; Pelusi, G. Persistence of increased uterine artery resistance in the third trimester and pregnancy outcome. Ultrasound Obstet. Gynecol. 2010, 36, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Unversucht, A.; Wessel, N.; Faber, R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007, 49, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Llurba, E.; Domínguez, C.; Martín-Gallán, P.; Cabero, L.; Gratacós, E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008, 31, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kusanovic, J.P.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Mittal, P.; Vaisbuch, E.; Mazaki-Tovi, S.; Gotsch, F.; Edwin, S.S.; Gomez, R.; et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J. Matern. Fetal Neonatal Med. 2009, 22, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Stubert, J.; Ullmann, S.; Bolz, M.; Külz, T.; Dieterich, M.; Richter, D.-U.; Reimer, T. Prediction of preeclampsia and induced delivery at <34 weeks gestation by sFLT-1 and PlGF in patients with abnormal midtrimester uterine Doppler velocimetry: A prospective cohort analysis. BMC Pregnancy Childbirth 2014, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Garcia-Tizon Larroca, S.; Peeva, G.; Poon, L.C.; Wright, D.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by serum placental growth factor and soluble fms-like tyrosine kinase-1 at 30–33 weeks’ gestation. Fetal Diagn. Ther. 2014, 35, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lisonkova, S.; Sabr, Y.; Mayer, C.; Young, C.; Skoll, A.; Joseph, K.S. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet. Gynecol. 2014, 124, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Bombrys, A.E.; Barton, J.R.; Nowacki, E.A.; Habli, M.; Pinder, L.; How, H.; Sibai, B.M. Expectant management of severe preeclampsia at less than 27 weeks’ gestation: Maternal and perinatal outcomes according to gestational age by weeks at onset of expectant management. Am. J. Obstet. Gynecol. 2008, 247, 247.e1–247.e6. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Kayem, G.; Tsatsaris, V.; Goffinet, F.; Sibai, B.M.; Haddad, B. Benefits and risks of expectant management of severe preeclampsia at less than 26 weeks gestation: The impact of gestational age and severe fetal growth restriction. Am. J. Obstet. Gynecol. 2011, 205, 465.e1–465.e6. [Google Scholar] [CrossRef] [PubMed]
- Manktelow, B.N.; Seaton, S.E.; Field, D.J.; Draper, E.S. Population-based estimates of in-unit survival for very preterm infants. Pediatrics 2013, 131, e425–e432. [Google Scholar] [CrossRef] [PubMed]
- Menzies, J.; Magee, L.A.; Li, J.; MacNab, Y.C.; Yin, R.; Stuart, H.; Baraty, B.; Lam, E.; Hamilton, T.; Lee, S.K.; et al. Instituting surveillance guidelines and adverse outcomes in preeclampsia. Obstet. Gynecol. 2007, 110, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.; Sennström, M.; Olovsson, M.; Brennecke, S.; Stepan, H.; Allegranza, D.; et al. Prediction of short-term outcome in pregnant women with suspected preeclampsia: The PROGNOSIS study. COGI 2014, Paris. Abstract P79. Available online: http://www.congressmed.com/cogi/images/pdf/COGIParisAbstractBook.pdf (accessed on 11 August 2015).
- Rana, S.; Karumanchi, S.A.; Lindheimer, M.D. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension 2014, 63, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, W.; Dukhovny, D.; Wenger, J.; Salahuddin, S.; Ralston, S.; Rana, S. Cost and resource implications with serum angiogenic factor estimation in the triage of pre-eclampsia. BJOG 2013, 120, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, W.; Rana, S.; Stepan, H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP síndrome. J. Perinat. Med. 2013, 41, 511–516. [Google Scholar] [PubMed]
- Dröge, L.; Herraìz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Venkatesha, S.; DePaepe, M.; Chien, E.K.; Paglia, M.; Karumanchi, S.A. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet. Gynecol. 2007, 109, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Bdolah, Y.; Palomaki, G.E.; Yaron, Y.; Bdolah-Abram, T.; Goldman, M.; Levine, R.J.; Sachs, B.P.; Haddow, J.E.; Karumanchi, S.A. Circulating angiogenic proteins in trisomy 13. Am. J. Obstet. Gynecol. 2006, 194, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Llurba, E.; Sánchez, O.; Ferrer, Q.; Nicolaides, K.H.; Ruíz, A.; Domínguez, C.; Sánchez-de-Toledo, J.; García-García, B.; Soro, G.; Arévalo, S.; Goya, M.; et al. Maternal and foetal angiogenic imbalance in congenital heart defects. Eur. Heart J. 2014, 35, 701–707. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herraiz, I.; Simón, E.; Gómez-Arriaga, P.I.; Martínez-Moratalla, J.M.; García-Burguillo, A.; Jiménez, E.A.L.; Galindo, A. Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration. Int. J. Mol. Sci. 2015, 16, 19009-19026. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819009
Herraiz I, Simón E, Gómez-Arriaga PI, Martínez-Moratalla JM, García-Burguillo A, Jiménez EAL, Galindo A. Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration. International Journal of Molecular Sciences. 2015; 16(8):19009-19026. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819009
Chicago/Turabian StyleHerraiz, Ignacio, Elisa Simón, Paula Isabel Gómez-Arriaga, José Manuel Martínez-Moratalla, Antonio García-Burguillo, Elena Ana López Jiménez, and Alberto Galindo. 2015. "Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration" International Journal of Molecular Sciences 16, no. 8: 19009-19026. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819009