Molecular and Functional Characterization of Thioredoxin 1from Korean Rose Bitterling (Rhodeus uyekii)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of R. uyekii Thioredoxin (RuTrx) the Nucleotide and Deduced Amino Acid Sequences

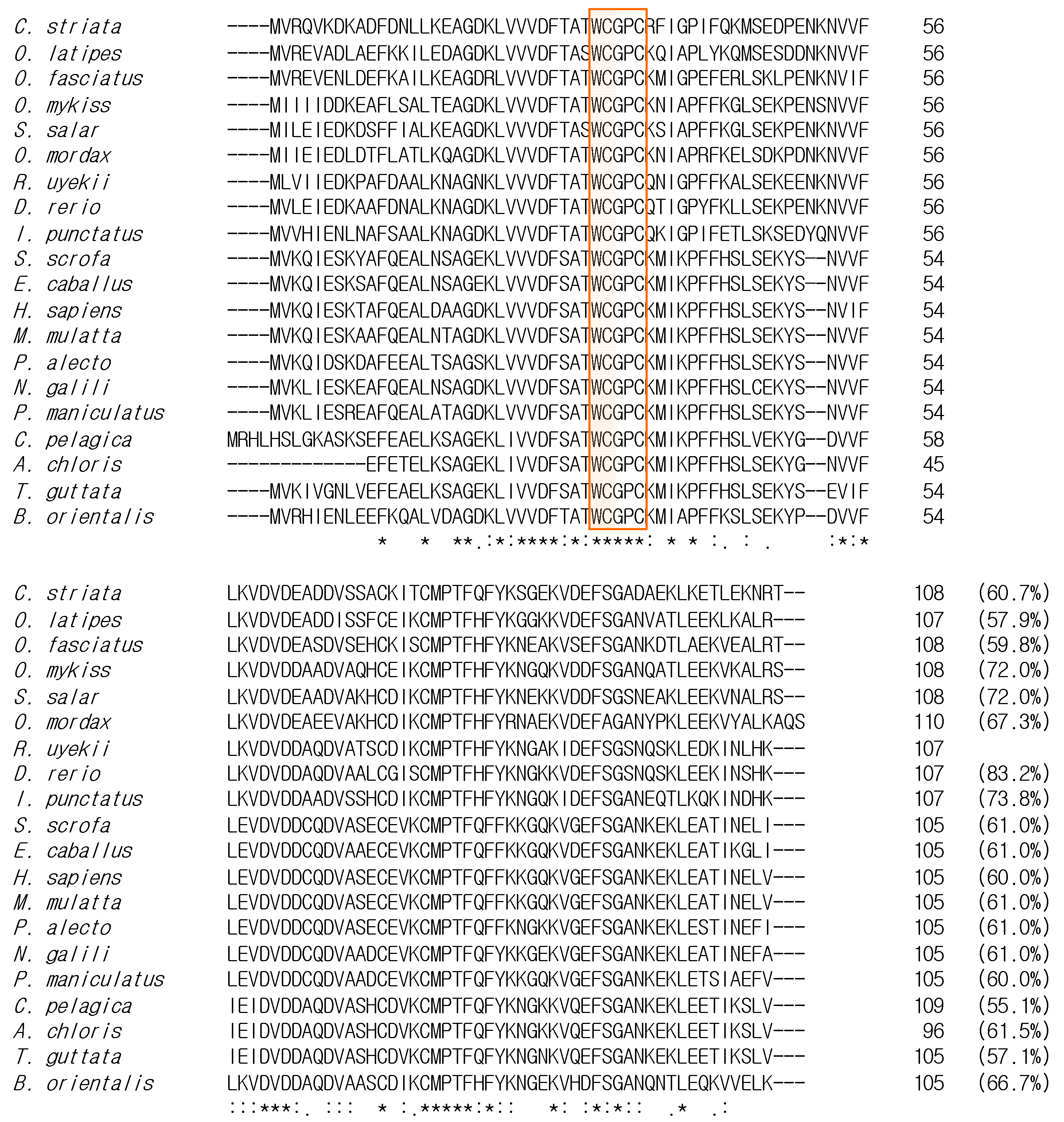
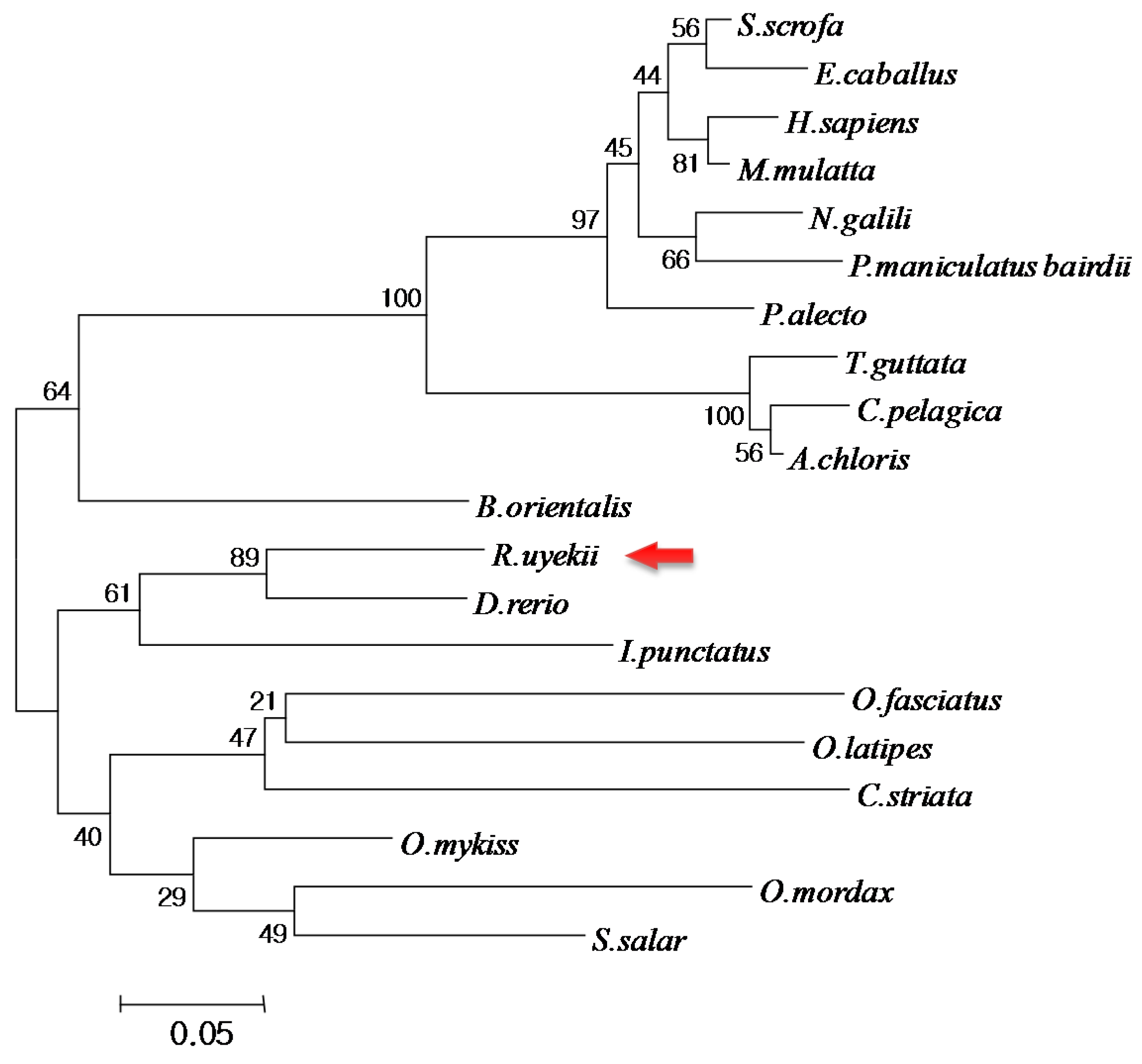
2.2. Comparison and Phylogenetic Analysis of RuTrx with Other Trx Homologs

2.3. Expression Analysis of RuTrx mRNA in Various Tissues and During Early Development

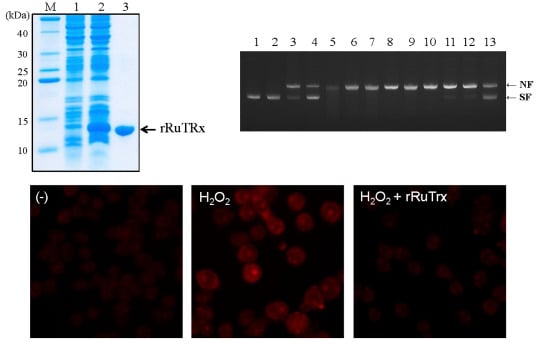
2.4. Overexpression and Purification of Recombinant RuTrx Protein
2.5. Antioxidant Activity of Recombinant RuTrx Protein against DNA Cleavage in Vitro
2.6. Antioxidant Activity of rRuTrx against ROS in Vivo
3. Experimental Section
3.1. Cloning of RuTrx from Rhoedeus uyekii
3.2. Multiple Sequence Alignment and Phylogenetic Analysis
3.3. Quantitative Real-Time PCR
3.4. Recombinant RuTrx Plasmid Construction
3.5. Expression and Purification of Recombinant RuTrx Protein
3.6. Animal and Tissue Preparation
3.7. DNA Cleavage Assay
3.8. Cell Culture
3.9. Detection of ROS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572–634. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.D.; Vitols, E. Thioredoxin from Lactobacillus leichmannii and its role as hydrogen donor for ribonucleoside triphosphate reductase. Biochem. Biophys. Res. Commun. 1966, 25, 109–115. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Spyrou, G.; Enmark, E.; Miranda-Vizuete, A.; Gustafsson, J. Cloning and expression of a novel mammalian thioredoxin. J. Biol. Chem. 1997, 272, 2936–2941. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Ljung, J.; Damdimopoulos, A.E.; Gustafsson, J.A.; Oko, R.; Pelto-Huikko, M.; Spyrou, G. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J. Biol. Chem. 2001, 276, 31567–31574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgren, A. Thioredoxin structure and mechanism: Conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 1995, 3, 239–243. [Google Scholar] [CrossRef]
- Revathy, K.S.; Umasuthan, N.; Lee, Y.; Whang, I.; Kim, H.C.; Lee, J. Cytosolic thioredoxin from Ruditapes philippinarum: Molecular cloning, characterization, expression and DNA protection activity of the recombinant protein. Dev. Comp. Immunol. 2012, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kwon, K.S.; Kim, S.R.; Rhee, S.G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998, 273, 15366–15372. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kim, W.J.; Kim, H.S.; Lee, Y.J.; Kim, C.H.; Nam, B.H.; Kim, Y.O.; Kim, D.G.; Lee, S.J.; Lim, S.G.; Kim, B.S. Molecular characterization of a tandem-repeat galectin-9 (RuGlec9) from Korean rose bitterling (Rhodeus uyekii). Fish Shellfish Immunol. 2012, 32, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Kim, E.M.; Kim, Y.J.; Lim, S.G.; Sim, D.S.; Kim, Y.H.; Park, I.S. Effect of lidocaine hydevrepochloride and clove oil as an anaes-thetic on Korean rose bitterling Rhodeus uyekii and oily bittering Acheilognathus koreensis. J. Aquac. 2005, 18, 272–279. [Google Scholar]
- Kim, B.C.; Kang, T.W.; Kim, M.S.; Kim, C.B. The complete mitogenome of Rhodeus uyekii (Cypriniformes, Cyprinidae). DNA Seq. 2006, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Shin, E.H.; Kong, H.J.; Kim, H.S.; Kim, B.S.; Nam, B.H.; Kim, Y.O.; Kim, C.H.; Jung, H.; An, C.M. Characterization of novel microsatellite markers derived from Korean rose bitterling (Rhodeus uyekii) genomic library. Genet. Mol. Res. 2014, 13, 8147–8152. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kim, J.L.; Kim, W.J.; Kim, H.S.; Yeo, S.Y.; Park, J.Y.; An, C.M. Genomic cloning and promoter analysis of the β-actin gene from Korean rose bitterling (Rhodeus uyekii). Genes Genom. 2014, 36, 861–869. [Google Scholar] [CrossRef]
- Cho, H.K.; Kong, H.J.; Moon, J.Y.; Kim, J.D.; Kim, D.G.; Kim, W.J.; Nam, B.H.; Kim, Y.O.; Kim, H.S.; An, C.M.; Kim, B.S. Molecular characterization and expression analysis of a peroxiredoxin 1 cDNA from Korean rose bitterling (Rhodeus uyekii). Mol. Biol. Rep. 2014, 41, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Lee, I.K.; Kim, J.; Kim, W.J.; Kim, H.S.; Cho, W.S.; Kim, D.W.; Park, J.Y.; An, C.M. RNA-Seq-based transcriptome analysis of Korean rose bitterling (Rhodeus uyekii) exposed to synthetic estrogen 17-α-ethinylestradiol (EE2). Mar. Genom. 2015. [CrossRef] [PubMed]
- Collet, J.F.; Messens, J. Structure, function, and mechanism of thioredoxin proteins. Antioxid. Redox Signal. 2010, 13, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Serata, M.; Iino, T.; Yasuda, E.; Sako, T. Roles of thioredoxin and thioredoxin reductase in the resistance to oxidative stress in Lactobacillus casei. Microbiology 2012, 158, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ning, X.; Chen, L.; Pei, D.; Zhao, J.; Zhang, L.; Liu, X.; Wu, H. Responses of thioredoxin 1 and thioredoxin-related protein 14 mRNAs to cadmium and copper stresses in Venerupis philippinarum. Comp. Biochem. Physiol. C Toxicol. Pharm. 2011, 154, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.W.; Jeong, J.M.; Park, H.J.; Park, C.I. Molecular cloning and expression analysis of a thioredoxin from rock bream, Oplegnathus fasciatus, and biological activity of the recombinant protein. Fish Shellfish Immunol. 2011, 31, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Fitzl, G.; Mozet, C.; Martin, H.; Welt, K.; Wieland, E. Effect of age and hypoxia/reoxygenation on mRNA expression of antioxidative enzymes in rat liver and kidneys. Exp. Gerontol. 2002, 37, 1481–1487. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Lu, X.J.; Chen, J. Molecular cloning and characteristic analysis of a thioredoxin from Neobenedenia melleni. Afr. J. Biotechnol. 2012, 11, 7582–7591. [Google Scholar]
- Reckenfelderbaumer, N.; Ludemann, H.; Schmidt, H.; Steverding, D.; Krauth-Siegel, R.L. Identification and Functional Characterization of Thioredoxin from Trypanosoma brucei brucei. J. Biol. Chem. 2000, 275, 7547–7552. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic. Res. Commun. 1989, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C. New aspects in the free-radical chemistry of pyrimidine nucleobases. Free Radic. Res. Commun. 1987, 2, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Toaldo, C.; Pettazzoni, P.; Dianzani, M.U.; Barrera, G. The “two-faced” effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers (Basel) 2010, 2, 338–363. [Google Scholar] [CrossRef] [PubMed]
- Androwiki, A.C.; Camargo Lde, L.; Sartoretto, S.; Couto, G.K.; Ribeiro, I.M.; Verissimo-Filho, S.; Rossoni, L.V.; Lopes, L.R. Protein disulfide isomerase expression increases in resistance arteries during hypertension development. Effects on Nox1 NADPH oxidase signaling. Front. Chem. 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.; Klein, M.; Erdbrugger, W.; Droge, W.; Schulze-Osthoff, K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc. Natl. Acad. Sci. USA 1994, 91, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Jikimoto, T.; Nishikubo, Y.; Koshiba, M.; Kanagawa, S.; Morinobu, S.; Morinobu, A.; Saura, R.; Mizuno, K.; Kondo, S.; Toyokuni, S.; et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Moon, J.Y.; Kim, W.-J.; Kim, D.-G.; Nam, B.-H.; Kim, Y.-O.; Park, J.Y.; An, C.M.; Kong, H.J. Molecular and Functional Characterization of Thioredoxin 1from Korean Rose Bitterling (Rhodeus uyekii). Int. J. Mol. Sci. 2015, 16, 19433-19446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819433
Kim J, Moon JY, Kim W-J, Kim D-G, Nam B-H, Kim Y-O, Park JY, An CM, Kong HJ. Molecular and Functional Characterization of Thioredoxin 1from Korean Rose Bitterling (Rhodeus uyekii). International Journal of Molecular Sciences. 2015; 16(8):19433-19446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819433
Chicago/Turabian StyleKim, Julan, Ji Young Moon, Woo-Jin Kim, Dong-Gyun Kim, Bo-Hye Nam, Young-Ok Kim, Jung Youn Park, Cheul Min An, and Hee Jeong Kong. 2015. "Molecular and Functional Characterization of Thioredoxin 1from Korean Rose Bitterling (Rhodeus uyekii)" International Journal of Molecular Sciences 16, no. 8: 19433-19446. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819433