Polymer/Iron Oxide Nanoparticle Composites—A Straight Forward and Scalable Synthesis Approach
Abstract
:1. Introduction
2. Results and Discussion
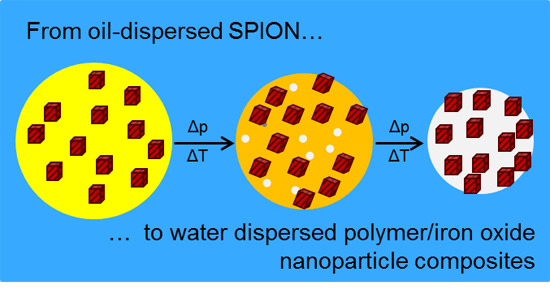
2.1. Preparation of Single-Core SPION and Multi-Core Nanocomposite Spheres

| Sample Code | Core | Polymer Matrix |
|---|---|---|
| A | Oleic acid stabilized iron oxide | poly(styrene) |
| B | Oleic acid stabilized iron oxide | poly(lactic acid) |
| C | Oleic acid stabilized iron oxide | poly(methyl methacrylate) |
| D | Oleic acid stabilized iron oxide | poly(caprolactone) |




2.2. Magnetic Characterization of Multicore Nanocomposite Spheres





| Sample | R1 (mmol−1·s−1) | R2 (mmol−1·s−1) | R2/R1 |
|---|---|---|---|
| Core nanocrystals | 9.1 | 61.9 | 6.8 |
| Sample A | 7.0 | 286.1 | 41.0 |
| Sample B | 4.6 | 285.9 | 61.7 |
| FeraSpin™-R | 10.4 | 185.2 | 17.8 |
| Resovist® [28] | 12.3 | 188 | 15.3 |
3. Experimental Section

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Costo, R.; Gruttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis methods to prepare single- and multi-core iron oxide nanoparticles for biomedical applications. Dalton Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, J.; Fornara, A.; Toprak, M.; Muhammed, M.; Kim, D.K. Synthesis and magnetic properties of bulk transparent pmma/fe-oxide nanocomposites. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Thaler, M.; Roy, S.; Fornara, A.; Bitsche, M.; Qin, J.; Muhammed, M.; Salvenmoser, W.; Rieger, G.; Fischer, A.S.; Glueckert, R. Visualization and analysis of superparamagnetic iron oxide nanoparticles in the inner ear by light microscopy and energy filtered tem. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Fornara, A.; Johansson, P.; Petersson, K.; Gustafsson, S.; Qin, J.; Olsson, E.; Ilver, D.; Krozer, A.; Muhammed, M.; Johansson, C. Tailored magnetic nanoparticles for direct and sensitive detection of biomolecules in biological samples. Nano Lett. 2008, 8, 3423–3428. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Asempah, I.; Laurent, S.; Fornara, A.; Muller, R.N.; Muhammed, M. Injectable superparamagnetic ferrogels for controlled release of hydrophobic drugs. Adv. Mater. 2009, 21, 1354–1357. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, W.; Poe, D.; Qin, J.; Fornara, A.; Zhang, Y.; Ramadan, U.A.; Muhammed, M.; Pyykkö, I. MRI manifestation of novel superparamagnetic iron oxide nanoparticles in the rat inner ear. Nanomedicine 2010, 5, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Fornara, A.; Qin, J.; Toprak, M.S.; Dalhammar, G.; Muhammed, M.; Rajarao, G.K. Characterization of superparamagnetic iron oxide nanoparticles and its application in protein purification. J. Nanosci. Nanotechnol. 2011, 11, 10201–10206. [Google Scholar] [CrossRef] [PubMed]
- Fornara, A.; Recalenda, A.; Qin, J.; Sugunan, A.; Ye, F.; Laurent, S.; Muller, R.N.; Zou, J.; Usama, A.-R.; Toprak, M.S.; et al. Polymeric/inorganic multifunctional nanoparticles for simultaneous drug delivery and visualization. MRS Proc. 2010, 1257. [Google Scholar] [CrossRef]
- Gustafsson, S.; Fornara, A.; Petersson, K.; Johansson, C.; Muhammed, M.; Olsson, E. Evolution of structural and magnetic properties of magnetite nanoparticles for biomedical applications. Cryst. Growth Des. 2010, 10, 2278–2284. [Google Scholar] [CrossRef]
- Ludwig, F.; Kazakova, O.; Barquín, L.F.; Fornara, A.; Trahms, L.; Steinhoff, U.; Svedlindh, P.; Wetterskog, E.; Pankhurst, Q.A.; Southern, P.; et al. Magnetic, structural, and particle size analysis of single- and multi-core magnetic nanoparticles. IEEE Trans. Magn. 2014, 50. [Google Scholar] [CrossRef]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T2 contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Elaissari, A. Organic-inorganic hybrid magnetic latex. Adv. Polym. Sci. 2010, 233, 237–281. [Google Scholar]
- Tanyolaç, D.; Özdural, A.R. New low cost magnetic material: Magnetic polyvinylbutyral microbeads. React Funct. Polym. 2000, 43, 279–286. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Faraj, A.A.; Canet-Soulas, E.; Bessueille, F.; Léonard, D.; Fessi, H. Elaboration of plla-based superparamagnetic nanoparticles: Characterization, magnetic behaviour study and in vitro relaxivity evaluation. Int. J. Pharm. 2007, 338, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Hamoudeh, M.; Fessi, H. Preparation, characterization and surface study of poly-epsilon caprolactone magnetic microparticles. J. Colloid Interface Sci. 2006, 300, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, J.R.; Shin, S.C.; Kim, J.C.; Chang, Y.H.; Lee, K.H.; Kim, J.D. Magnetic enhancement of iron oxide nanoparticles encapsulated with poly(d,l-latide-co-glycolide). Colloid Surf. A 2005, 255, 19–25. [Google Scholar] [CrossRef]
- Demortiere, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Begin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.M.; Khandhar, A.P.; Jonasson, C.; Blomgren, J.; Johansson, C.; Krishnan, K.M. Size-dependent relaxation properties of monodisperse magnetite nanoparticles measured over seven decades of frequency by ac susceptometry. IEEE Trans. Magn. 2013, 49, 3441–3444. [Google Scholar] [CrossRef] [PubMed]
- Öisjöen, F.; Schneiderman, J.F.; Astalan, A.P.; Kalabukhov, A.; Johansson, C.; Winkler, D. A new approach for bioassays based on frequency- and time-domain measurements of magnetic nanoparticles. Biosens. Bioelectron. 2010, 25, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Ahrentorp, F.; Astalan, A.P.; Jonasson, C.; Blomgren, J.; Qi, B.; Mefford, O.T.; Yan, M.; Courtois, J.; Berret, J.F.; Fresnais, J.; et al. Sensitive high frequency ac susceptometry in magnetic nanoparticle applications. AIP Conf. Proc. 2010, 1311, 213–223. [Google Scholar]
- Ludwig, F.; Guillaume, A.; Schilling, M.; Frickel, N.; Schmidt, A.M. Determination of core and hydrodynamic size distributions of coFe2O4 nanoparticle suspensions using ac susceptibility measurements. J Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Ahrentorp, F.; Astalan, A.; Blomgren, J.; Jonasson, C.; Wetterskog, E.; Svedlindh, P.; Lak, A.; Ludwig, F.; van Ijzendoorn, L.J.; Westphal, F.; et al. Effective particle magnetic moment of multi-core particles. J. Magn. Magn. Mater. 2015, 380, 221–226. [Google Scholar] [CrossRef]
- Wiekhorst, F.; Steinhoff, U.; Eberbeck, D.; Trahms, L. Magnetorelaxometry assisting biomedical applications of magnetic nanoparticles. Pharm. Res. 2012, 29, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, F.; Mäuselein, S.; Heim, E.; Schilling, M. Magnetorelaxometry of magnetic nanoparticles in magnetically unshielded environment utilizing a differential fluxgate arrangement. Rev. Sci. Instrum. 2005, 76, 106102. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Lu, J.; Xia, C.; Gao, F.; Gong, Q.; Song, B.; Zhao, X.; Shuai, X.; Chen, X.; et al. Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labeling and in vivo imaging. Biomaterials 2011, 32, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Balzer, T. Ferucarbotran (resovist): A new clinically approved res-specific contrast agent for contrast-enhanced mri of the liver: Properties, clinical development, and applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [PubMed]
- Yathindranath, V.; Rebbouh, L.; Moore, D.F.; Miller, D.W.; Lierop, J.V.; Hegmann, T. A versatile method for the reductive, one-pot synthesis of bare, hydrophilic and hydrophobic magnetite nanoparticles. Adv. Mater. 2011, 21, 1457–1464. [Google Scholar]
- Chantrell, R.W.; Hoon, S.H.; Tanner, B.K. Time-dependent magnetization in fine-particle ferromagnetic systems. J. Magn. Magn. Mater. 1983, 38, 133–141. [Google Scholar] [CrossRef]
- Romanus, E.; Berkov, D.V.; Prass, S.; Groβ, C.; Weitschies, W.; Weber, P. Determination of energy barrier distributions of magnetic nanoparticles by temperature dependent magnetorelaxometry. Nanotechnology 2003, 14, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Eberbeck, D.; Wiekhorst, F.; Steinhoff, U.; Trahms, L. Aggregation behaviour of magnetic nanoparticle suspensions investigated by magnetorelaxometry. J. Phys. Condens. Matter 2006, 18, S2829–S2846. [Google Scholar] [CrossRef]
- Ludwig, F.; Heim, E.; Schilling, M. Characterization of superparamagnetic nanoparticles by analyzing the magnetization and relaxation dynamics using fluxgate magnetometers. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommertune, J.; Sugunan, A.; Ahniyaz, A.; Bejhed, R.S.; Sarwe, A.; Johansson, C.; Balceris, C.; Ludwig, F.; Posth, O.; Fornara, A. Polymer/Iron Oxide Nanoparticle Composites—A Straight Forward and Scalable Synthesis Approach. Int. J. Mol. Sci. 2015, 16, 19752-19768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819752
Sommertune J, Sugunan A, Ahniyaz A, Bejhed RS, Sarwe A, Johansson C, Balceris C, Ludwig F, Posth O, Fornara A. Polymer/Iron Oxide Nanoparticle Composites—A Straight Forward and Scalable Synthesis Approach. International Journal of Molecular Sciences. 2015; 16(8):19752-19768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819752
Chicago/Turabian StyleSommertune, Jens, Abhilash Sugunan, Anwar Ahniyaz, Rebecca Stjernberg Bejhed, Anna Sarwe, Christer Johansson, Christoph Balceris, Frank Ludwig, Oliver Posth, and Andrea Fornara. 2015. "Polymer/Iron Oxide Nanoparticle Composites—A Straight Forward and Scalable Synthesis Approach" International Journal of Molecular Sciences 16, no. 8: 19752-19768. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819752