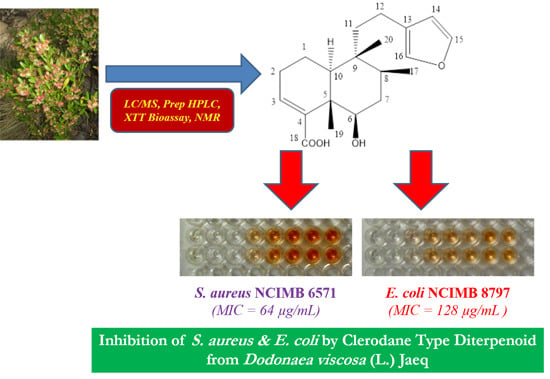
Rapid Bioassay-Guided Isolation of Antibacterial Clerodane Type Diterpenoid from Dodonaea viscosa (L.) Jaeq.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fractionation of Plant Material
2.2. Preliminary Antibacterial Screening
| D. viscosa | Test Bacteria | |
|---|---|---|
| Fraction | E. coli (NCIMB 8797) | S. aureus (NCIMB 6571) |
| Hexane (H) | 12.5 ± 0.5 | 11.4 ± 0.5 |
| Dichloromethane (D) | 0.0 | 0.0 |
| Ethyl acetate (E) | 9.7 ± 0.3 | 9.0 ± 0.2 |
| Butanol (B) | 0.0 | 10.0 ± 0.2 |
| Aqueous (A) | 0.0 | 0.0 |
| Ciprofloxacin | 31.0 ± 0.5 | – |
| Clarithromycin | – | 28.6 ± 0.5 |
2.3. Optimization of XTT (2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) Bioassay
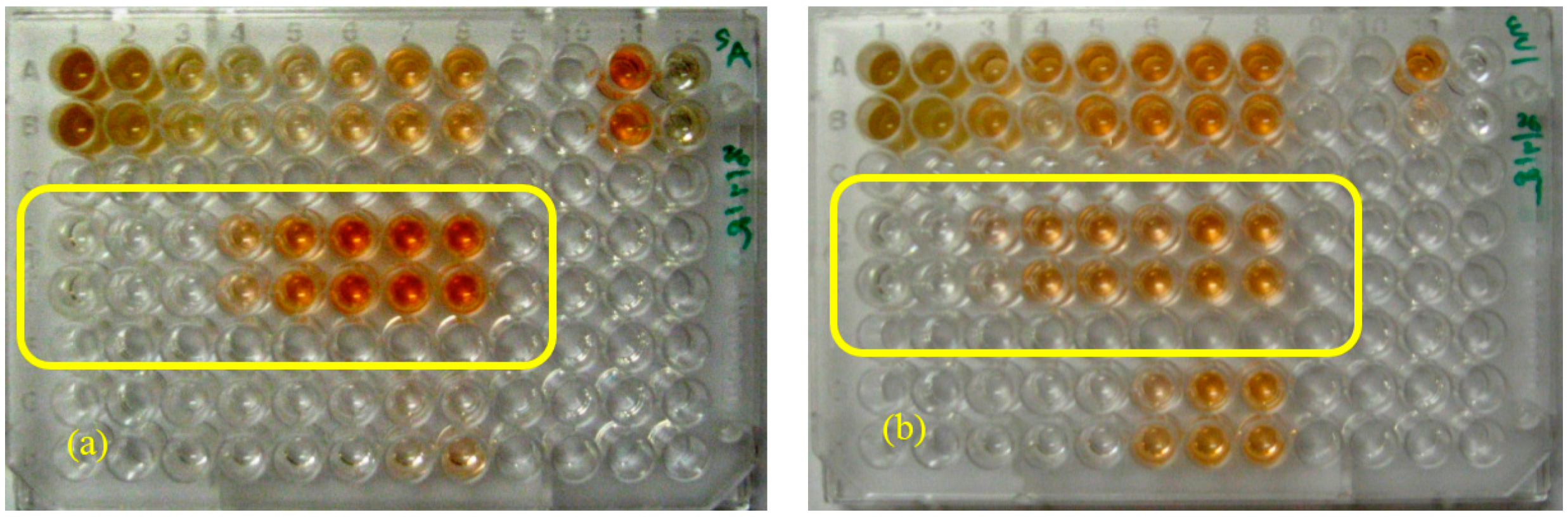
2.4. Generic Preparative HPLC-XTT Bioassay
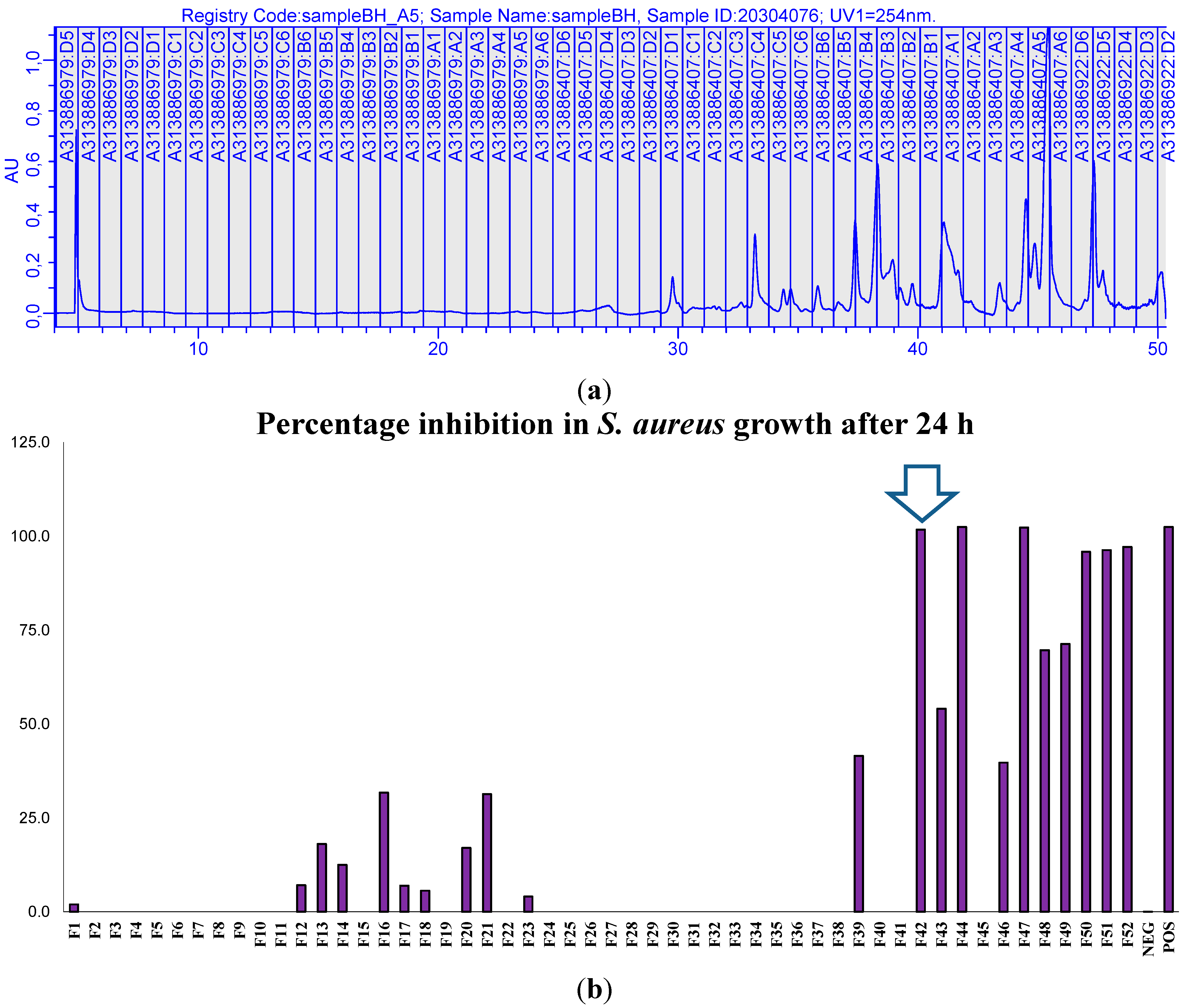
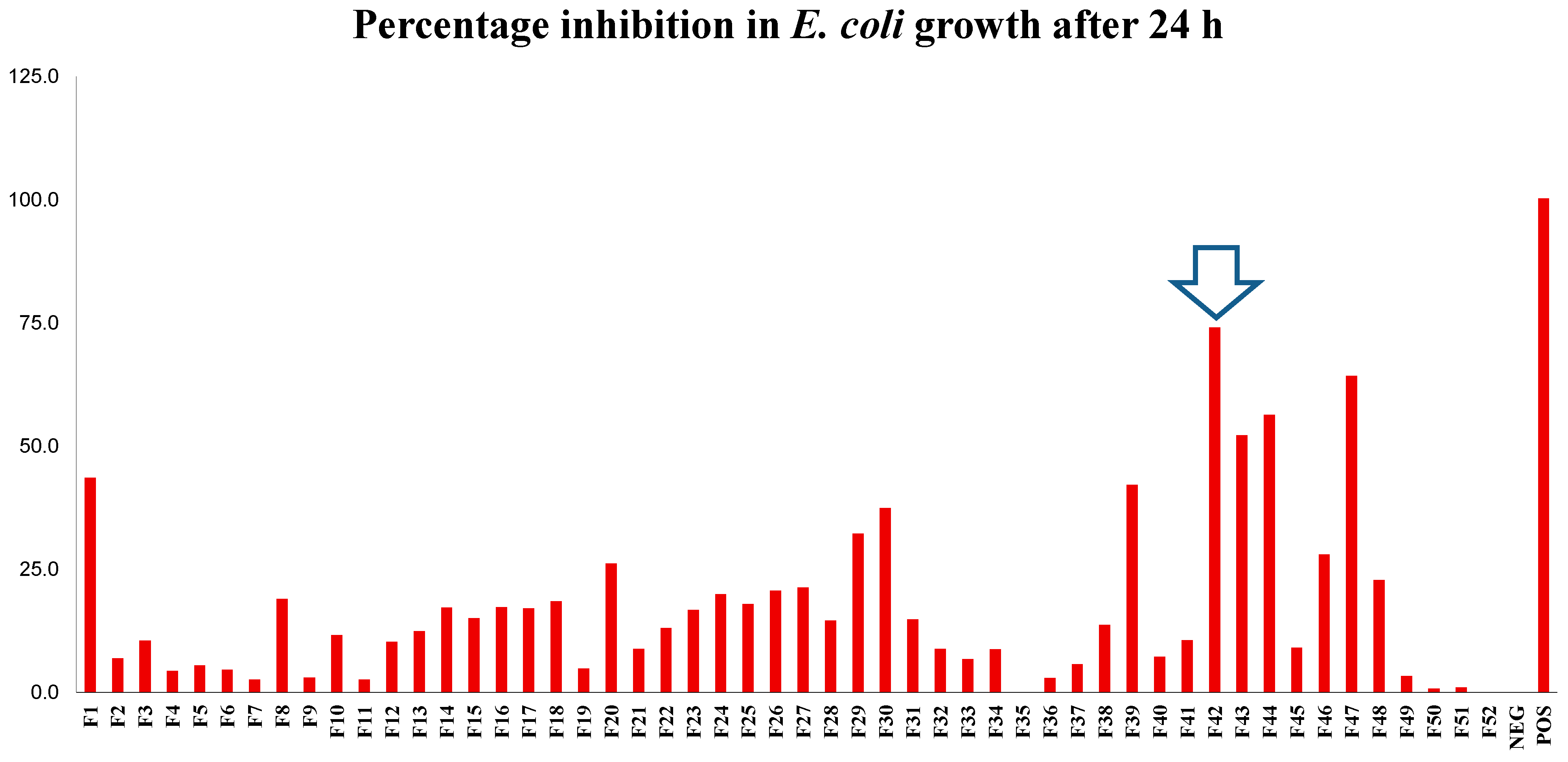
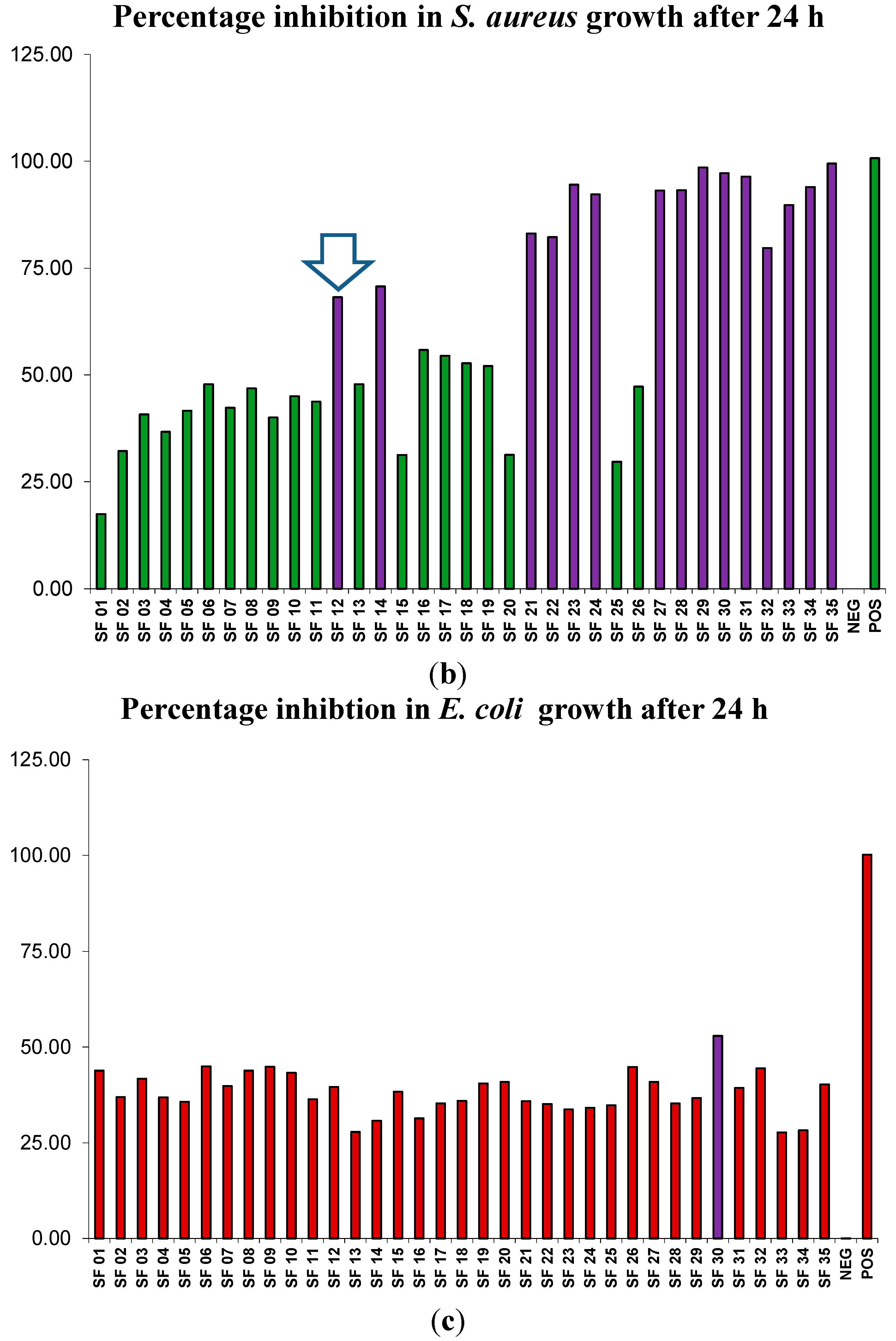
2.5. Focused Gradients-XTT Bioassay
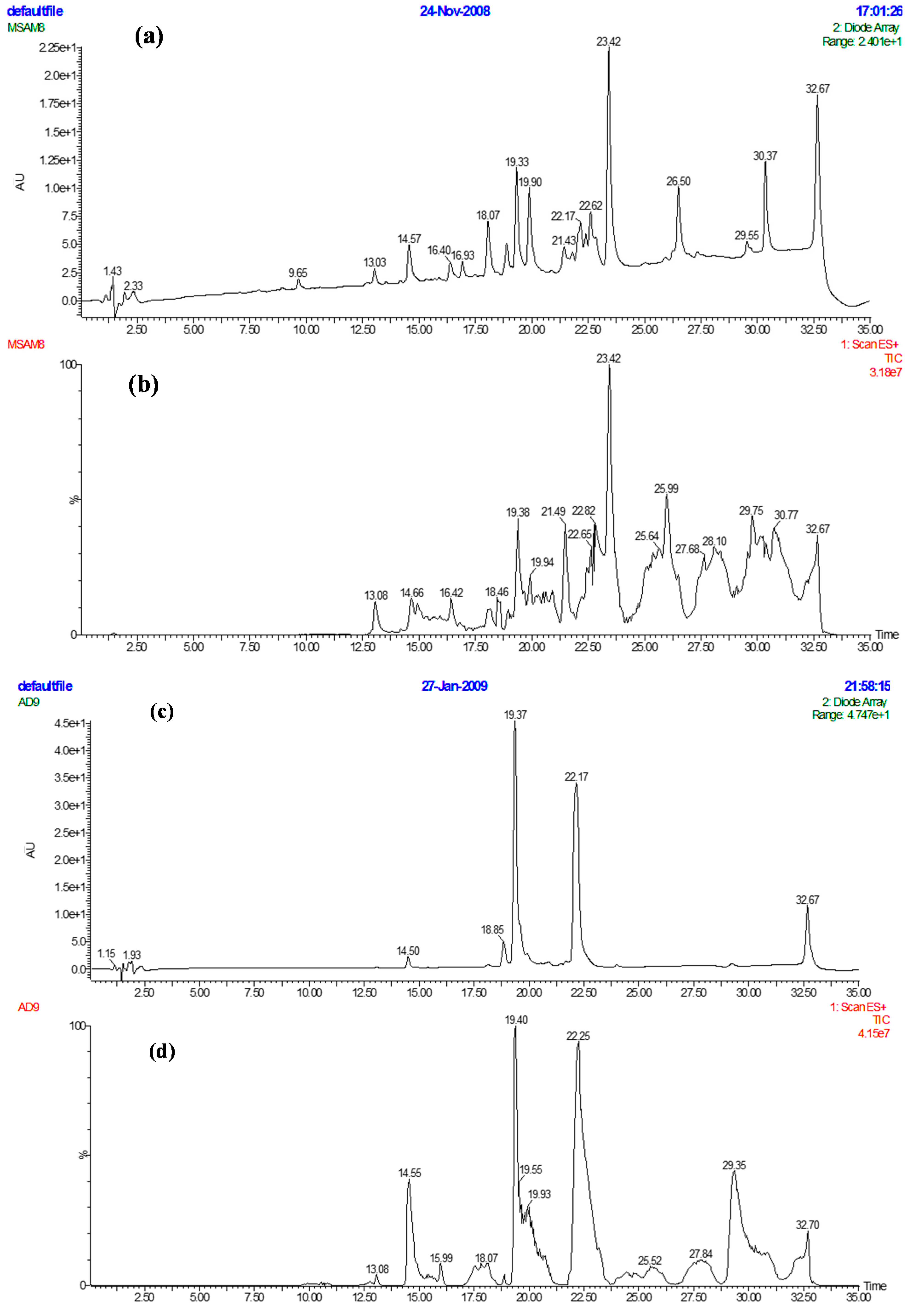
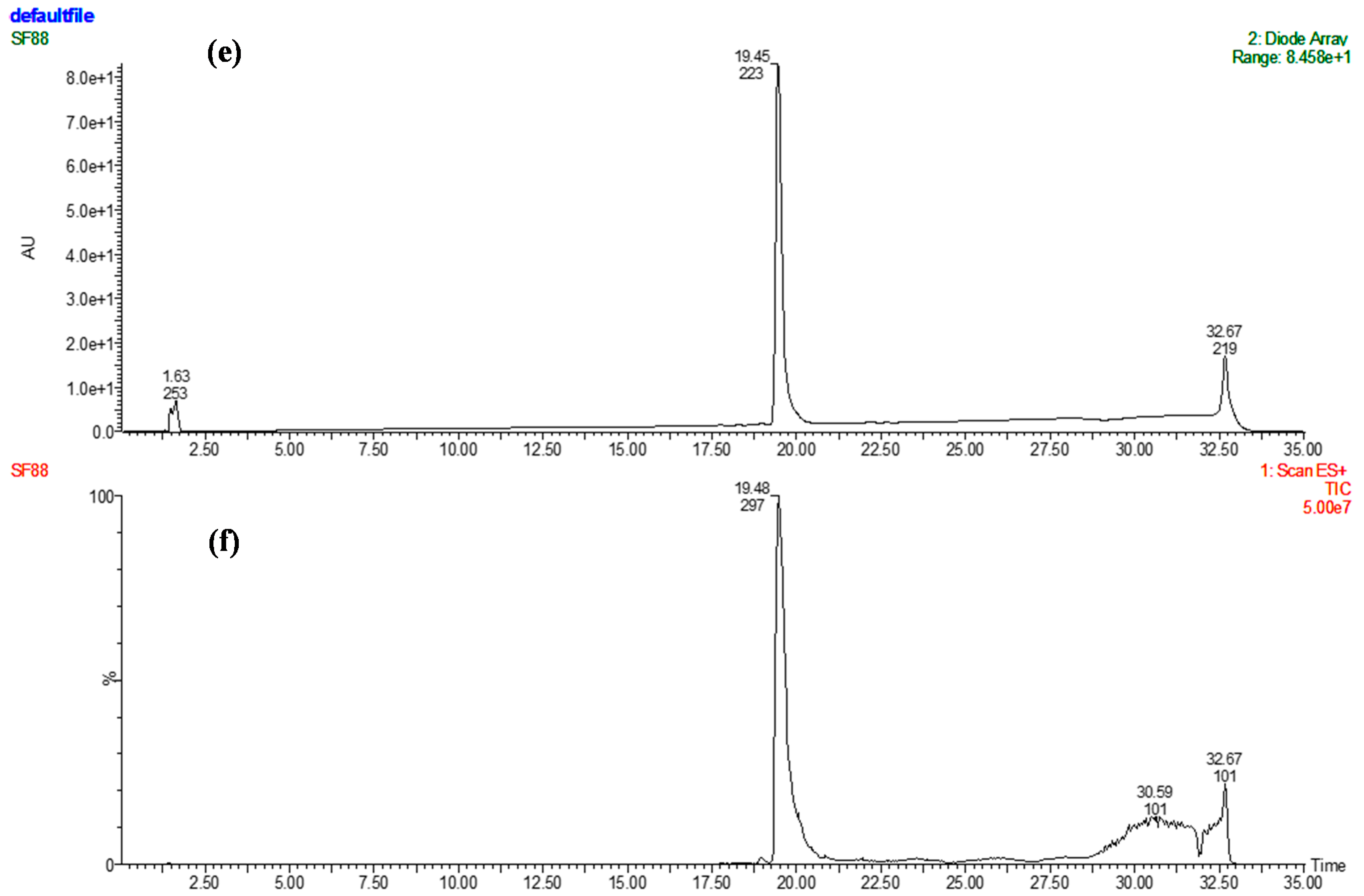
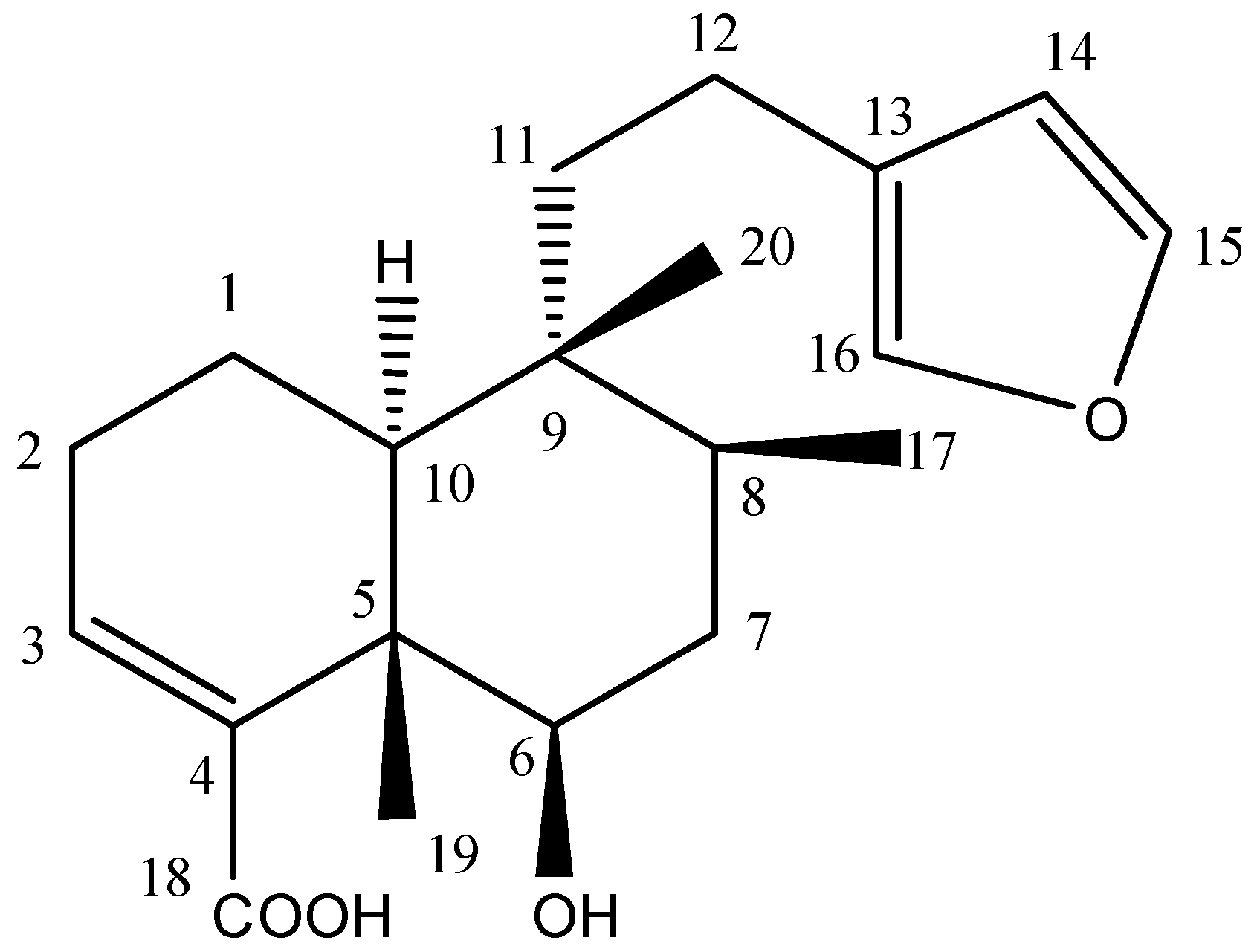
| C. No | 13C-NMR (δ) | Multiplicity DEPT | 1H-NMR (δ) | 1H/13C (HMBC) Connectivity |
|---|---|---|---|---|
| 1 | 17.5 | CH2 | α1.75 (m) β1.65 (m) | H-3 (6.86) |
| 2 | 27.4 | CH2 | 2.33 (m) | H-3 (6.86), H-10 (1.40) |
| 3 | 140.6 | CH | 6.86 (dd, 2.9, 4.6) | H-2 (2.33), Hα-1 (1.75), Hβ-1 (1.65) |
| 4 | 141.9 | C | – | H-3 (6.86), H-6 (3.64), H-17 (1.22) |
| 5 | 45.1 | C | – | H-3 (6.86), H-17 (1.22) |
| 6 | 75.2 | CH | 3.64 (dd, 5.1, 10.8) | H-17 (1.22) |
| 7 | 36.4 | CH2 | 1.60 (m) | – |
| 8 | 34.4 | CH | 1.76 (m) | H-18 (0.87), H-19 (0.76) |
| 9 | 39.3 | C | – | H-18 (0.87) |
| 10 | 46.2 | CH | 1.40 (bd, 12.0) | H-19 (0.76) |
| 11 | 39.0 | CH2 | 1.61 (m) | H-10 (1.40), H-20 (0.76) |
| 12 | 18.0 | CH2 | 2.30 (m) | H-19 (0.76) |
| 13 | 125.9 | C | – | H-12 (2.30), H-14 (6.30), H-15 (7.38), H-16 (7.27) |
| 14 | 111.1 | CH | 6.30 (bs) | H-15 (7.38), H-16 (7.27) |
| 15 | 143.2 | CH | 7.38 (t, 1.8) | H-14 (6.30), H-16 (7.27) |
| 16 | 139.0 | CH | 7.27 (bs) | H-12 (2.30), H-14 (6.30), H-15 (7.38) |
| 17 | 15.2 | CH3 | 0.87 (d, 6.8) | – |
| 18 | 174.1 | C | – | – |
| 19 | 16.1 | CH3 | 1.22 (s) | H-6 (3.64) |
| 20 | 17.4 | CH3 | 0.76 (s) | – |
2.6. MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) of Compound 1
| Bacterial Susceptibility Assays | Compound 1 | Clarithromycin | Ciprofloxacin | |||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | S. aureus | E. coli | |
| MIC (μg/mL) | 64 | 128 | 25 | – | – | 25 |
| MBC (μg/mL) | 128 | 256 | 50 | – | – | 50 |
3. Experimental Section
3.1. Chemicals
3.2. Bacterial Strains
3.3. Plant Material and Extraction Procedures

3.4. Chemical Characterization
3.4.1. Preparative HPLC
| Sample—Fraction (mg Dry Weight) | Percentage Methanol | Focused Gradient |
|---|---|---|
| D. viscosa—B18 a (1.0) | 42 | 25%–55% methanol |
| D. viscosa—B19 (2.2) | 43 | |
| D. viscosa—B20 (5.9) | 45 | |
| D. viscosa—H42 b (1.9) | 83 | 80%–100% |
| D. viscosa—H44 (2.9) | 86 | |
| D. viscosa—H47 (1.6) | 91 | |
| D. viscosa—H50 (1.1) | 97 | |
| D. viscosa—H51 (1.0) | 98 | |
| D. viscosa—H52 (1.6) | 100 | 80%–100% and hold for 10 min |
Focused Separation of Bioactive Fractions
| Sample—Primary Fraction | Sub-Fraction |
|---|---|
| D. viscosa—H42 | 12, 14, 21–24, 29, 30 |
| D. viscosa—H44 | 15, 17 |
| D. viscosa—H50 | 25–27 |
| D. viscosa—H51 | 27–30 |
| D. viscosa—H52 | 21, 23, 24, 37, 38 |
3.4.2. Analytical HPLC
3.4.3. Physical and Spectral Data for Compound 1
3.5. Antibacterial Activity
3.5.1. Preliminary Antibacterial Screening
3.5.2. XTT Bioassay
Determination of Minimum Inhibitory Concentration (MIC) of Purified Compound
3.5.3. Determination of Minimum Bactericidal Concentration (MBC) of Purified Compound
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Arun, M.; Asha, V.V. Gastroprotective effect of Dodonaea viscosa on various experimental ulcer models. J. Ethanopharmacol. 2008, 118, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Maharaj, J.V.; Smith, J.P. Investigating South African plants as a source of new antimalarial drugs. J. Ethanopharmacol. 2008, 119, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, D.; Nathan, S.; Suresh, T.; Perumalsamy, L.P. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J. Ethanopharmacol. 2001, 74, 217–220. [Google Scholar] [CrossRef]
- Getie, M.; Gebre-Mariam, T.; Rietz, R.; Hohne, C.; Huschka, C.; Schmidtke, M.; Abate, A.; Neubert, H.H.R. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia 2003, 74, 139–143. [Google Scholar] [CrossRef]
- Patel, M.; Coogan, M.M. Antifungal activity of the plant Dodonaea viscosa var. angustifolia on Candida albicans from HIV-infected patients. J. Ethanopharmacol. 2008, 118, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Hernandez, L.; Pereda-Miranda, R.; Mata, R. Screening for antimicrobial activity of crude drug extracts and pure natural products from Mexican medicinal plants. J. Ethanopharmacol. 1992, 35, 275–283. [Google Scholar] [CrossRef]
- Eldridge, G.R.; Vervoot, H.C.; Lee, C.M.; Cremin, P.A.; Williams, C.T.; Hart, S.M.; Goering, M.G.; O’Neil-Johnson, M.; Zeng, L. High-throughput method for the production and analysis of large natural product libraries for drug discovery. Anal. Chem. 2002, 74, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Jeffries, C.; Ruan, H.; Nelson, C.; Smithson, D.; Shelat, A.A.; Brown, K.M.; Li, X.; Hester, J.P.; Smillie, T.; et al. Automated high-throughput system to fractionate plant natural products for drug discovery. J. Nat. Prod. 2010, 73, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, J.; Hart, M.; Jarelöv, A.; Kühn, I.; McKenzie, D.; Mölby, R. Evaluation of redox indicators and the use of digital scanners and spectrophotometer for quantification of microbial growth in microplates. J. Microbiol. Methods 2002, 50, 63–73. [Google Scholar] [CrossRef]
- Williams, C.; Espinosa, O.A.; Montenegro, H.; Cubilla, L.; Capson, T.L.; Ortega-Barria, E.; Romero, L.I. Hydrosoluble formazan XTT: Its application to natural products drug discovery for Leishmania. J. Microbiol. Methods 2003, 55, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Al-Bakri, G.A.; Afifi, U.F. Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J. Microbiol. Methods 2007, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Anis, I.; Anis, E.; Ahmed, S.; Mustafa, G.; Malik, A.; Amtul, Z.; Atta-ur-Rahman. Thormbin inhibitory constituents from Duranta repens. Helv. Chim. Acta 2001, 84, 649–655. [Google Scholar] [CrossRef]
- Geis, W.; Buschauer, B.; Becker, H. cis-Clerodandes from axenic cultures of the liverwort Scapania nemorea. Phytochemistry 1999, 51, 643–649. [Google Scholar] [CrossRef]
- McCrindle, R.; Nakamura, E.; Anderson, B.A. Constituents of Solidago species. Part VII. Constitution and stereochemistry of the cis-clerodanes from Solidago arguta Ait. and of related diterpenoids. J. Chem. Soc. Perkins Trans. 1976, 1, 1590–1597. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; King, M.R. Diterpenes and norditerpenes from the Aristeguetia group. Phytochemistry 1991, 30, 2991–3000. [Google Scholar] [CrossRef]
- Das, B.; Reddy, R.M.; Ramu, R.; Ravindranath, N.; Harish, H.; Ramakrishna, S.V. K.; Rao, K.Y.; Harakishore, K.; Murthy, N.S.U. Clerodane diterpenoids from Pulicaria wightiana. Phytochemistry 2005, 66, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Brehm-Stecher, F.B.; Johnson, A.E. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed]
- Khurram, M.; Khan, A.M.; Hameed, A.; Abbas, N.; Qayum, A.; Inayat, H. Antibacterial activities of Dodonaea viscosa using Contact Bioautography Technique. Molecules 2009, 14, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.B.; Sahm, F.D.; Weissfeld, S.A. Bailey & Scott’s Diagnostic Microbiology, 10th ed.; Elsevier: St. Louis, MO, USA, 2007; p. 188. [Google Scholar]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Khurram, M.; Lawton, L.A.; Edwards, C.; Khan, F.A.; Naseemullah; Shah, Z.U.; Hameed, A. Evaluation of antibacterial potential of Quercus baloot using a rapid bioassay-guided approach. Middle East J. Sci. Res. 2013, 13, 273–279. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurram, M.; Lawton, L.A.; Edwards, C.; Iriti, M.; Hameed, A.; Khan, M.A.; Khan, F.A.; Rahman, S.U. Rapid Bioassay-Guided Isolation of Antibacterial Clerodane Type Diterpenoid from Dodonaea viscosa (L.) Jaeq. Int. J. Mol. Sci. 2015, 16, 20290-20307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160920290
Khurram M, Lawton LA, Edwards C, Iriti M, Hameed A, Khan MA, Khan FA, Rahman SU. Rapid Bioassay-Guided Isolation of Antibacterial Clerodane Type Diterpenoid from Dodonaea viscosa (L.) Jaeq. International Journal of Molecular Sciences. 2015; 16(9):20290-20307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160920290
Chicago/Turabian StyleKhurram, Muhammad, Linda A. Lawton, Christine Edwards, Marcello Iriti, Abdul Hameed, Murad A. Khan, Farman A. Khan, and Shafiq Ur Rahman. 2015. "Rapid Bioassay-Guided Isolation of Antibacterial Clerodane Type Diterpenoid from Dodonaea viscosa (L.) Jaeq." International Journal of Molecular Sciences 16, no. 9: 20290-20307. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160920290