Drug Carrier for Photodynamic Cancer Therapy
Abstract
:1. Introduction
1.1. Principle and Mechanism of Photodynamic Therapy


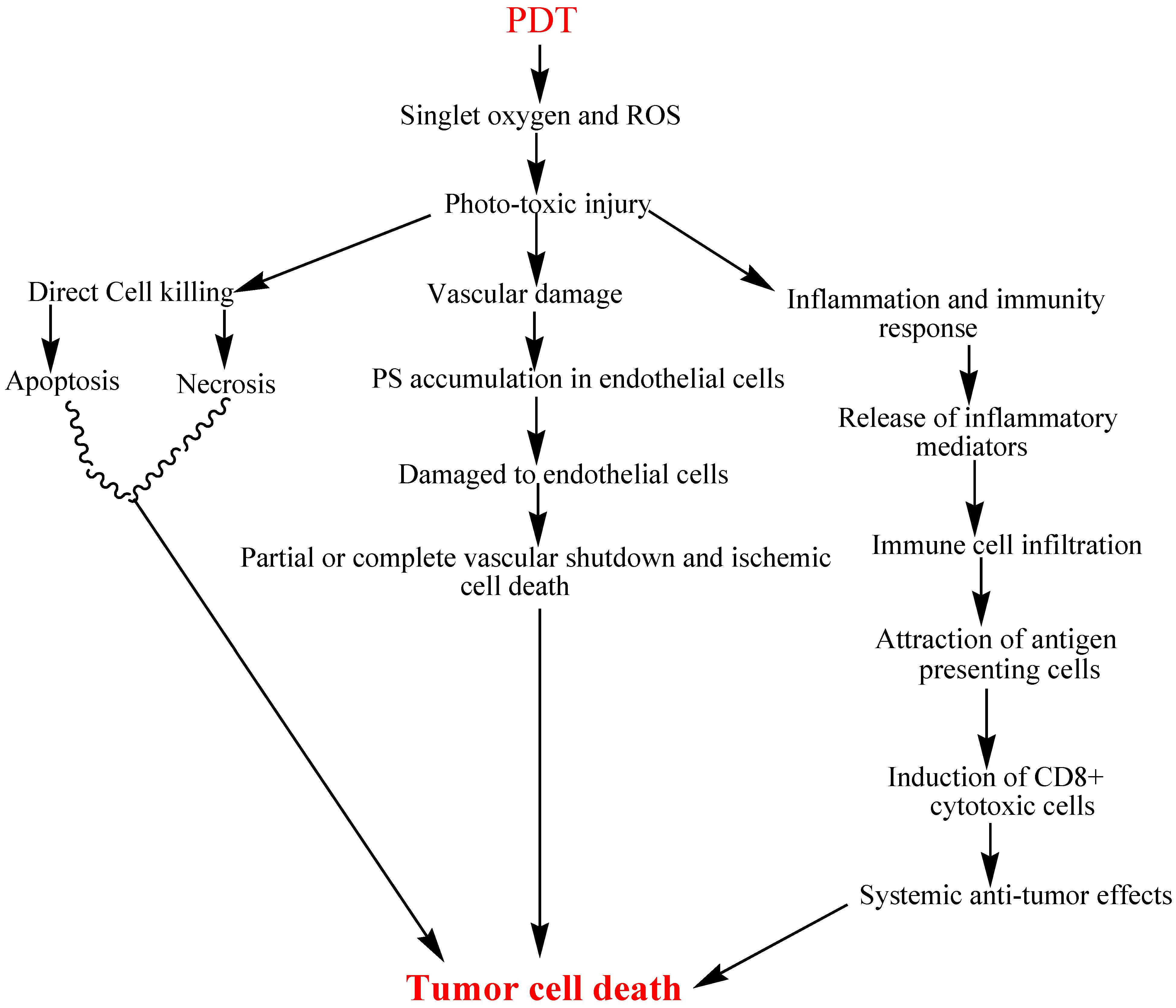
1.2. Anti-Tumor Activity of PDT
1.2.1. Direct Tumor Cell Kill
1.2.2. Vascular Damage
1.2.3. Inflammatory and Immune Response
1.3. Photosensitizer
| Photosensitizer | Approved Application |
|---|---|
| Porfimer sodium (Photofrin) | Used in the treatment of early and late-stage lung cancers, esophageal cancer, bladder cancer, early stage cervical cancer, and malignant and nonmalignant skin diseases. It is also being considered as potential therapy against Kaposi’s sarcoma, Barrett’s esophagus with high-grade dysplasia, psoriasis, and cancers of the head, brain, neck and breast [45,46] |
| 5-Aminolevulinic acid or ALA (Levulan) | US FDA approved for non-oncological PDT treatment of actinic keratosis in 1999. Its potential PDT applications extend to Bowen’s disease, basal cell carcinoma, and other diseases. ALA can also be used to detect tumors in bladder, skin, lung, and gastrointestinal tract [47,48,49,50] |
| Methyl aminolevulinate (Metvixia) | Approved by the US FDA in 2004 for treatment of actinic keratosis [51,52] |
| Meta tetra(hydroxyphenyl) chlorin (Foscan) | Treatment of neck and scalp cancer with m-THPC was approved in Europe, and the drug was used successfully for the treatment of breast, prostate, and pancreatic cancers [53,54,55] |
| N-aspartyl chlorin e6 (NPe6, Laserphyrin) | Approved for the treatment of fibrosarcoma, liver cancer, brain cancer, and oral cancer. Approved in Japan in 2003 to treat lung cancer [46] |
| Benzoporphyrin derivative monoacid ring A (Visudyne) | In 1999, US FDA approved Visudyne for age-related macular degeneration in ophthalmology [41] |
| n-Hexyl ester of ALA (Cysview) | Approved in 2010 by the US FDA for the diagnosis of bladder cancer [56] |
| Photosensitizers | Potential indication |
|---|---|
| Hypocrellin A | White lesions of vulva and keloid cases, antiviral activity against human immunodeficiency virus type 1 and age-related macular degeneration [57,58] |
| Pheophorbide-a | Early stage lung cancer, superficial head and neck cancer and human uterine cancer [59,60] |
| Chlorin e6 | Superficial squamous cell carcinoma of the lung, human nasopharyngeal and bladder carcinomas [61,62,63] |
| Methylene blue | Basal cell carcinoma, Kaposi’s sarcoma and Melanoma [64,65] |
| Hypericin | Bladder cancer, nasopharyngeal carcinoma cells [66,67] |
| Phthalocyanine | Cutaneous/subcutaneous lesions from diverse solid tumor origins [68] |
| Rose Bengal | Metastatic melanoma [69] |
| HPPH: 2-(1-Hexyl-oxyethyl)-2-devinyl pyropheophorbide-alpha | Equine periocular squamous cell carcinoma, rodent colon carcinoma and xenografts of human glioma [70,71] |
1.4. Light Source in PDT
2. Photosensitizer Delivery
2.1. Nanoparticles-Based PSs Delivery in PDT
3. Organic Nanoparticles
3.1. Liposomes
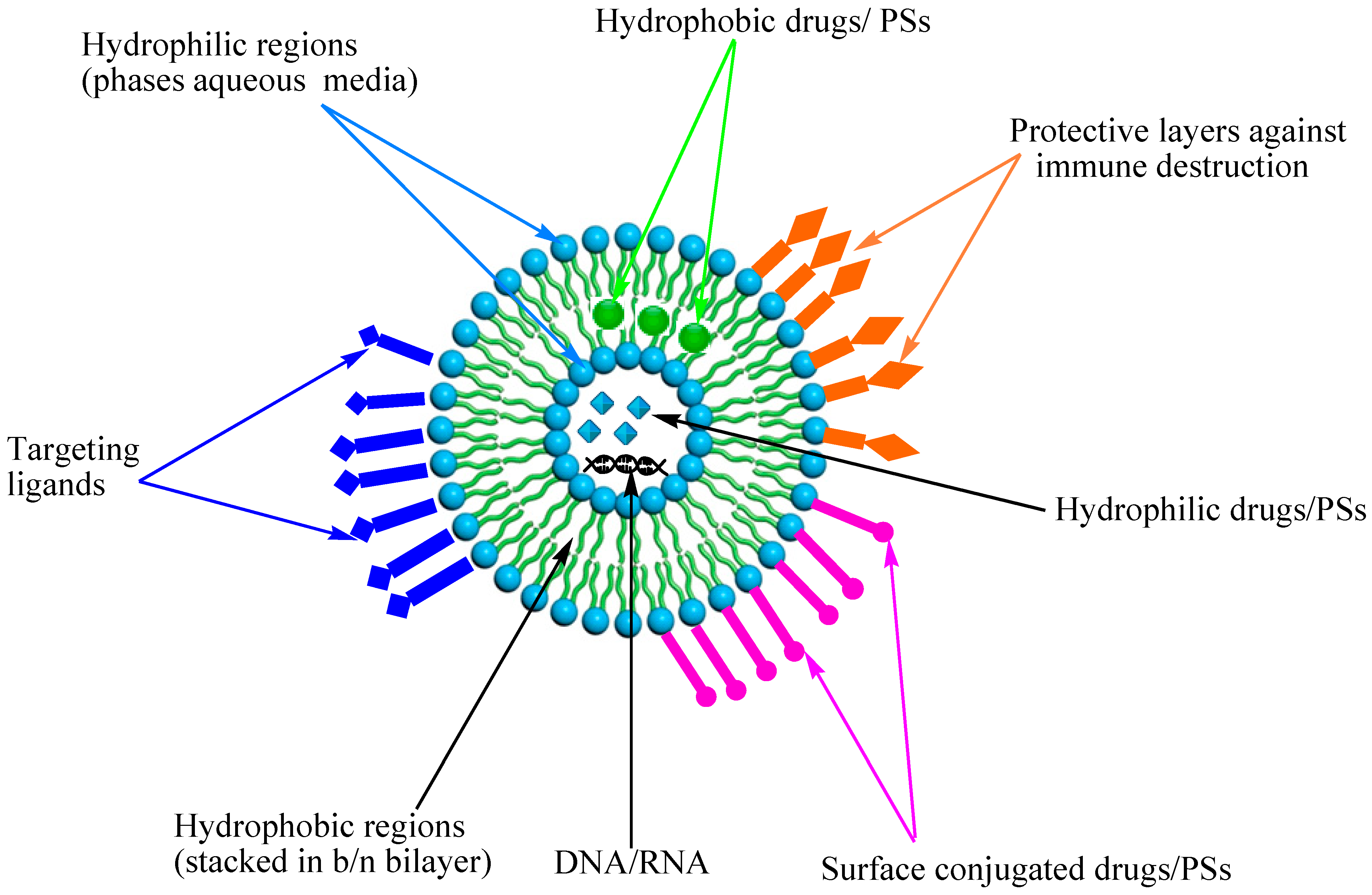
3.2. Polymeric Nanoparticles
3.2.1. Natural Polymeric Nanoparticles
Albumin
Chitosan
Hyaluronic Acid
3.2.2. Synthetic Polymer
PS loading of Polymeric Micelle
Mechanisms of PS Release from Polymers
- (i)
- Enzymatic reaction that results in cleavage or degradation of the polymer at the site of delivery, thereby releasing PSs from the core.
- (ii)
- Swelling of polymeric nanoparticles due to hydration, pH and temperature, followed by release through diffusion.
- (iii)
- Dissociation and de-adsorption of the drug from the polymer through a concentration gradient.
Homopolymers
Block Copolymer
Graft Copolymer
Dendrimers and Hyperbranched Polymer
Hydrogel
4. Inorganic Nanocarriers
4.1. Drug Loading and Release from Inorganic Nanocarriers
4.2. Quantum dots


4.3. Ceramic-Based Nanoparticles
4.4. Metallic Nanoparticles
4.5. Carbon Materials
5. Side Effects of PDT
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Komerik, N.; Wilson, M.; Poole, S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 2000, 72, 676–680. [Google Scholar] [CrossRef]
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Szeimies, R.M.; Jori, G.; Abels, C. Antibacterial photodynamic therapy in dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, L. Photogeneration of singlet oxygen (1O2) and free radicals (Sen−, O−2) by tetra-brominated hypocrellin B derivative. Free Radic. Res. 2001, 35, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Staicu, A.; Pascu, A.; Nuta, A.; Sorescu, A.; Raditoiu, V.; Pascu, M. Studies about phthalocyanine photosensitizers to be used in photodynamic therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. [Google Scholar]
- Zhao, B.; He, Y.Y. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev. Anticancer Ther. 2010, 10, 1797–809. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, M.A. Photodynamic therapy in dermatology: An update on applications and outcomes. Semin. Cutan. Med. Surg. 2008, 27, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Abels, C. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT). Photochem. Photobiol. Sci. 2004, 3, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Nowis, D.; Makowski, M.; Stoklosa, T.; Legat, M.; Issat, T.; Golab, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.; Villanueva, A.; Stockert, J.C.; Canete, M. Regulated necrosis in HeLa cells induced by ZnPc photodynamic treatment: A new nuclear morphology. Int. J. Mol. Sci. 2014, 15, 22772–22785. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [PubMed]
- Kessel, D.; Vicente, M.G.; Reiners, J.J. Initiation of apoptosis and autophagy by photodynamic therapy. Lasers Surg. Med. 2006, 38, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Carvalho, A.P.; Duarte, C.B. Intracellular signaling mechanisms in photodynamic therapy. BBA Gen. Subj. 2004, 1704, 59–86. [Google Scholar] [CrossRef] [PubMed]
- Bittremieux, M.; Bultynck, G. P53 and Ca2+ signaling from the endoplasmic reticulum: Partners in anti-cancer therapies. Oncoscience 2015, 2, 233–238. [Google Scholar] [PubMed]
- Acedo, P.; Zawacka-Pankau, J. P53 family members—Important messengers in cell death signaling in photodynamic therapy of cancer? Photochem. Photobiol. Sci. 2015, 14, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Bonora, M.; Missiroli, S.; Poletti, F.; Ramirez, F.G.; Morciano, G.; Morganti, C.; Pandolfi, P.P.; Mammano, F.; Pinton, P. Intravital imaging reveals p53-dependent cancer cell death induced by phototherapy via calcium signaling. Oncotarget 2015, 6, 1435–1445. [Google Scholar] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H.; Wieman, T.J.; Wiehle, S.A.; Cerrito, P.B. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992, 52, 4914–4921. [Google Scholar] [PubMed]
- Kerdel, F.A.; Soter, N.A.; Lim, H.W. In vivo mediator release and degranulation of mast cells in hematoporphyrin derivative-induced phototoxicity in mice. J. Investig. Dermatol. 1987, 88, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Fingar, V.H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987, 47, 3110–3114. [Google Scholar] [PubMed]
- Chen, B.; Pogue, B.W.; Luna, J.M.; Hardman, R.L.; Hoopes, P.J.; Hasan, T. Tumor vascular permeabilization by vascular-targeting photosensitization: Effects, mechanism, and therapeutic implications. Clin. Cancer Res. 2006, 12, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Milla Sanabria, L.; Rodriguez, M.E.; Cogno, I.S.; Rumie Vittar, N.B.; Pansa, M.F.; Lamberti, M.J.; Rivarola, V.A. Direct and indirect photodynamic therapy effects on the cellular and molecular components of the tumor microenvironment. BBA-Res. Cancer 2013, 1835, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Cecic, I.; Korbelik, M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002, 183, 43–51. [Google Scholar] [CrossRef]
- Korbelik, M.; Cecic, I. Complement activation cascade and its regulation: Relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B Biol. 2008, 93, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar] [PubMed]
- Li, F.; Cheng, Y.; Lu, J.; Hu, R.; Wan, Q.; Feng, H. Photodynamic therapy boosts anti-glioma immunity in mice: A dependence on the activities of T cells and complement C3. J. Cell. Biochem. 2011, 112, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Moan, J. Properties for optimal PDT sensitizers. J. Photochem. Photobiol. B Biol. 1990, 5, 521–524. [Google Scholar] [CrossRef]
- Ormond, A.; Freeman, H. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Schuitmaker, J.J.; Baas, P.; van Leengoed, H.L.; van der Meulen, F.W.; Star, W.M.; van Zandwijk, N. Photodynamic therapy: A promising new modality for the treatment of cancer. J. Photochem. Photobiol. B Biol. 1996, 34, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Stylli, S.S.; Kaye, A.H.; MacGregor, L.; Howes, M.; Rajendra, P. Photodynamic therapy of high grade glioma—Long Term survival. J. Clin. Neurosci. 2005, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in photodynamic therapy—A search for ideal photosensitizers. Curr. Med. Chem. Anti-Cancer Agent. 2002, 2, 187–207. [Google Scholar] [CrossRef]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Batlle, A.M. Porphyrins, porphyrias, cancer and photodynamic therapy—A model for carcinogenesis. J. Photochem. Photobiol. B Biol. 1993, 20, 5–22. [Google Scholar] [CrossRef]
- Dougherty, T.J. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002, 20, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Brown, S.B.; Collins, S.; Ibbotson, S.; Jenkinson, H.; Kurwa, H.; Langmack, K.; McKenna, K.; Moseley, H.; Pearse, A.D.; et al. Guidelines for topical photodynamic therapy: Report of a workshop of the British Photodermatology Group. Br. J. Dermatol. 2002, 146, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, H.I.; Kim, M.N.; Kim, B.J.; Chun, Y.J.; Kim, D. Topical photodynamic therapy with methyl aminolevulinate may be an alternative therapeutic option for the recalcitrant Malassezia folliculitis. Int. J. Dermatol. 2011, 50, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A. Methyl aminolevulinate: Actinic keratoses and Bowen’s disease. Dermatol. Clin. 2007, 25, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan(R), 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)—A second-generation photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A. Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005, 81, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, W.; Liu, Q.; Nakagawa, H.; Sakaki, H.; Teh, B.; Matsumiya, T.; Yoshida, H.; Imaizumi, T.; Satoh, K.; Kimura, H. Photodynamic therapy with mono-L-aspartyl chlorin e6 can cause necrosis of squamous cell carcinoma of tongue: Experimental study on an animal model of nude mouse. Oral Oncol. 2006, 42, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-H.; Cai, Y.-J.; Liao, X.-R.; Wu, K.; Wang, L.; Zhang, D.-B.; Meng, Q. Isolation and identification of a new hypocrellin A-producing strain Shiraia sp. SUPER-H168. Microbiol. Res. 2009, 164, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Zhou, J.; Chen, J.; Harris, L.; Yip, L.; Towers, G.H.N. Hypocrellin, from hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 1994, 60, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Liu, X.Z.; Zhang, D.M.; Fong, W.P.; Fung, K.P. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial-mediated pathway in human uterine carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Busch, T.; Cengel, K.A.; Finlay, J. Pheophorbide a as a photosensitizer in photodynamic therapy: In vivo considerations. Cancer Biol. Ther. 2009, 8, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Lee, L.S.; Thong, P.S.P.; Olivo, M.; Chin, W.W.L.; Ramaswamy, B.; Kho, K.W.; Lim, P.L.; Lau, W.K.O. Chlorin e6-polyvinylpyrrolidone mediated photodynamic therapy—A potential bladder sparing option for high risk non-muscle invasive bladder cancer. Photodiagn. Photodyn. Ther. 2010, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.W.L.; Heng, P.W.S.; Thong, P.S.P.; Bhuvaneswari, R.; Hirt, W.; Kuenzel, S.; Soo, K.C.; Olivo, M. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur. J. Pharm. Biopharm. 2008, 69, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Samy, N.A.; Salah, M.M.; Ali, M.F.; Sadek, A.M. Effect of methylene blue-mediated photodynamic therapy for treatment of basal cell carcinoma. LasersMed. Sci. 2015, 30, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Saw, C.L.; Heng, P.W.; Olivo, M. Potentiation of the photodynamic action of hypericin. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Yang, S.; Wang, C.; Fu, X.; Wang, J.; Mao, Y.; Zhang, J.; Li, Y. Cellular and molecular mechanisms of photodynamic hypericin therapy for nasopharyngeal carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, T.J.; Baron, E.D.; Colussi, V.C.; Cooper, K.D.; Hoppel, C.L.; Ingalls, S.T.; Kenney, M.E.; Li, X.; Oleinick, N.L.; Stevens, S.R.; et al. Preliminary Clinical and Pharmacologic Investigation of Photodynamic Therapy with the Silicon Phthalocyanine Photosensitizer Pc 4 for Primary or Metastatic Cutaneous Cancers. Front. Oncol. 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W. American Society of Clinical Oncology, 2010 Annual Meeting and Rose Bengal: From a Wool Dye to a Cancer Therapy. Pharm. Ther. 2010, 35, 469–478. [Google Scholar]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Lobel, J.; MacDonald, I.J.; Ciesielski, M.J.; Barone, T.; Potter, W.R.; Pollina, J.; Plunkett, R.J.; Fenstermaker, R.A.; Dougherty, T.J. 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: Implications for photodynamic therapy. Lasers Surg. Med. 2001, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wu, H.; Zheng, Y.; Xu, X.; Yu, J. The effect of noncoherent red light irradiation on proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Lasers Med. Sci. 2012, 27, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Sibata, C.; Colussi, V.; Oleinick, N.; Kinsella, T. Photodynamic therapy: A new concept in medical treatment. Braz. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.S. Lasers and light sources for PDT: Past, present and future. Photodiagn. Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef]
- Mizeret, J.C.; van den Bergh, H.E. Cylindrical fiberoptic light diffuser for medical applications. Lasers Surg. Med. 1996, 19, 159–167. [Google Scholar] [CrossRef]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Prabhakaran, M.P.; Rodriguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, H.P.; Wagner, M.; Bezdetnaya, L.; Guillemin, F.; Schneckenburger, H. Fluorescence imaging of Foscan and Foslip in the plasma membrane and in whole cells. J. Photochem. Photobiol. B Biol. 2008, 92, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Olivo, M.; Bhuvaneswari, R.; Lucky, S.S.; Dendukuri, N.; Soo-Ping Thong, P. Targeted therapy of cancer using photodynamic therapy in combination with multi-faceted anti-tumor modalities. Pharmaceuticals 2010, 3, 1507–1529. [Google Scholar] [CrossRef]
- Sharman, W. Targeted photodynamic therapy via receptor mediated delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, D.; Foran, P.; MacMahon, P.; Shelly, M.J.; Eustace, S.; O’Kennedy, R. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv. Drug Deliv. Rev. 2009, 61, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.B.; Freeman, H.S. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Hussain, N. Transcytosis of nanoparticle and dendrimer delivery systems: Evolving vistas. Adv. Drug Deliv. Rev. 2001, 50, 69–89. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002, 66, 89–106. [Google Scholar] [CrossRef]
- Mitragotri, S.; Stayton, P. Organic nanoparticles for drug delivery and imaging. MRS Bull. 2014, 39, 219–223. [Google Scholar] [CrossRef]
- López-Dávila, V.; Seifalian, A.M.; Loizidou, M. Organic nanocarriers for cancer drug delivery. Curr. Opin. Pharmacol. 2012, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 2010, 6, 4026–4044. [Google Scholar] [CrossRef]
- Jiang, F.; Lilge, L.; Grenier, J.; Li, Y.; Wilson, M.D.; Chopp, M. Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg. Med. 1998, 22, 74–80. [Google Scholar] [CrossRef]
- Jiang, F.; Lilge, L.; Logie, B.; Li, Y.; Chopp, M. Photodynamic therapy of 9L gliosarcoma with liposome-delivered photofrin. Photochem. Photobiol. 1997, 65, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lilge, L.; Wilson, B.C. Photodynamic therapy of intracranial tissues: A preclinical comparative study of four different photosensitizers. J. Clin. Laser Med. Surg. 1998, 16, 81–91. [Google Scholar] [PubMed]
- Cattel, L.; Ceruti, M.; Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. Tumori 2002, 89, 237–249. [Google Scholar] [CrossRef]
- Woodle, M.C. Surface-modified liposomes: assessment and characterization for increased stability and prolonged blood circulation. Chem. Phys. Lipids 1993, 64, 249–262. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Iwasaki, F.; Sugiyama, I.; Horiuchi, K.; Hirano, T.; Ozawa, H.; Kanayama, N.; Oku, N. Phototoxicity of coproporphyrin as a novel photodynamic therapy was enhanced by liposomalization. Toxicol. Lett. 2008, 182, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; He, Y.Y.; Huang, C.G.; Huang, J.S.; Huang, Y.C.; An, J.Y.; Gu, Y.; Jiang, L.J. Pharmacokinetics, tissue distribution and photodynamic therapy efficacy of liposomal-delivered hypocrellin A, a potential photosensitizer for tumor therapy. Photochem. Photobiol. 1999, 70, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Derycke, A.S.L.; de Witte, P.A.M. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Namiki, Y.; Namiki, T.; Date, M.; Yanagihara, K.; Yashiro, M.; Takahashi, H. Enhanced photodynamic antitumor effect on gastric cancer by a novel photosensitive stealth liposome. Pharmacol. Res. 2004, 50, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kepczynski, M.; Nawalany, K.; Jachimska, B.; Romek, M.; Nowakowska, M. Pegylated tetraarylporphyrin entrapped in liposomal membranes. A possible novel drug-carrier system for photodynamic therapy. Colloids Surf. B Biointerfaces 2006, 49, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nawalany, K.; Rusin, A.; Kepczynski, M.; Mikhailov, A.; Kramer-Marek, G.; Snietura, M.; Poltowicz, J.; Krawczyk, Z.; Nowakowska, M. Comparison of photodynamic efficacy of tetraarylporphyrin pegylated or encapsulated in liposomes: In vitro studies. J. Photochem. Photobiol. B Biol. 2009, 97, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Nawalany, K.; Rusin, A.; Kepczynski, M.; Filipczak, P.; Kumorek, M.; Kozik, B.; Weitman, H.; Ehrenberg, B.; Krawczyk, Z.; Nowakowska, M. Novel nanostructural photosensitizers for photodynamic therapy: In vitro studies. Int. J. Pharm. 2012, 430, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, L.A.; Joanitti, G.A.; Silva, J.R.; Longo, J.P.F.; Azevedo, R.B. Liposomal photosensitizers: Potential platforms for anticancer photodynamic therapy. Braz. J. Med. Biol. Res. 2011, 44, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Bressler, S.B. Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Chakravarthi, S.S.; Robinson, D.H.; De, S. Nanoparticles prepared using natural and synthetic polymers. In Nanoparticulate Drug Delivery Systems; Thassu, D., Deleers, M., Pathak, Y.V., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Volume 3, p. 51. [Google Scholar]
- Wang, S.; Fan, W.; Kim, G.; Hah, H.J.; Lee, Y.E.; Kopelman, R.; Ethirajan, M.; Gupta, A.; Goswami, L.N.; Pera, P.; et al. Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers Surg. Med. 2011, 43, 686–695. [Google Scholar] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, A.; Amareshwar, P.; Chakravarty, P. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–50. [Google Scholar]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Bugaj, A.M. Targeted photodynamic therapy—A promising strategy of tumor treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huo, M.; Zhou, J.; Zhang, Y.; Peng, X.; Yu, D.; Zhang, H.; Li, J. Synthesis, characterization, drug-loading capacity and safety of novel octyl modified serum albumin micelles. Int. J. Pharm. 2009, 376, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Chen, K.; Preuss, A.; Possemeyer, K.; Roeder, B.; Langer, K. Photosensitizer loaded HSA nanoparticles. I: Preparation and photophysical properties. Int. J. Pharm. 2010, 393, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wacker, M.; Hackbarth, S.; Ludwig, C.; Langer, K.; Roder, B. Photophysical evaluation of mTHPC-loaded HSA nanoparticles as novel PDT delivery systems. J. Photochem. Photobiol. B Biol. 2010, 101, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Preuss, A.; Hackbarth, S.; Wacker, M.; Langer, K.; Roder, B. Novel photosensitizer-protein nanoparticles for photodynamic therapy: Photophysical characterization and in vitro investigations. J. Photochem. Photobiol. B Biol. 2009, 96, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Sharma, S.K.; Dai, T.; Chung, H.; Yaroslavsky, A.; Garcia-Diaz, M.; Chang, J.; Chiang, L.Y.; Hamblin, M.R. Can nanotechnology potentiate photodynamic therapy? Nanotechnol. Rev. 2012, 1, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ghadi, A.; Mahjoub, S.; Tabandeh, F.; Talebnia, F. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Casp. J. Int. Med. 2014, 5, 156–161. [Google Scholar]
- Zhang, L.; Dou, S.; Li, Y.; Yuan, Y.; Ji, Y.; Wang, Y.; Yang, Y. Degradation and compatibility behaviors of poly(glycolic acid) grafted chitosan. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.Y.; Kwon, S.H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, K.; Oh, Y.K.; Kwon, S.H.; Her, S.; Kim, I.S.; Choi, K.; Lee, S.J.; Kim, H.; Lee, S.G.; et al. Tumor specificity and therapeutic efficacy of photosensitizer-encapsulated glycol chitosan-based nanoparticles in tumor-bearing mice. Biomaterials 2009, 30, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Koo, H.; Jeong, H.; Huh, M.S.; Choi, Y.; Jeong, S.Y.; Byun, Y.; Choi, K.; Kim, K.; Kwon, I.C. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J. Control. Release 2011, 152, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.C.; Na, K. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials 2010, 31, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.H.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.K.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Galus, R.; Antiszko, M.; Wlodarski, P. Clinical applications of hyaluronic acid. Pol. Merkur. Lekarski 2006, 20, 606–608. [Google Scholar] [PubMed]
- Williams, D.L.; Mann, B.K. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement: A masked controlled study. PLoS ONE 2014, 9, e99766. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Parks, W.T.; Wight, T.N. Intracellular hyaluronan in arterial smooth muscle cells: Association with microtubules, RHAMM, and the mitotic spindle. J. Histochem. Cytochem. 2004, 52, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chen, C.H.; Shalumon, K.; Chen, J.P. Preparation and characterization of antiadhesion barrier film from hyaluronic acid-grafted electrospun poly(caprolactone) nanofibrous membranes for prevention of flexor tendon postoperative peritendinous adhesion. Int. J. Nanomed. 2014, 9, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.; Walker, M.; Waddington, R.; Chen, W. Comparison of the antioxidant properties of wound dressing materials–carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte-derived reactive oxygen species. Biomaterials 2003, 24, 1549–1557. [Google Scholar] [CrossRef]
- Evanich, J.D.; Evanich, C.J.; Wright, M.B.; Rydlewicz, J.A. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin. Orthop. Relat. Res. 2001, 390, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Koo, H.; Choi, K.Y.; Lee, S.J.; Kim, K.; Kwon, I.C.; Leary, J.F.; Park, K.; Yuk, S.H.; Park, J.H.; et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials 2012, 33, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Prestwich, G.D. Synthesis and Selective Cytotoxicity of a Hyaluronic Acid−Antitumor Bioconjugate. Bioconjug. Chem. 1999, 10, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bae, B.-C.; Na, K. Acetylated Hyaluronic Acid/Photosensitizer Conjugate for the Preparation of Nanogels with Controllable Phototoxicity: Synthesis, Characterization, Autophotoquenching Properties, and in vitro Phototoxicity against HeLa Cells. Bioconjug. Chem. 2010, 21, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Neuse, E.W. Synthetic polymers as drug-delivery vehicles in medicine. Metal-Based Drugs 2008, 2008, 469531. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Del. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sant, V.P.; Smith, D.; Leroux, J.-C. Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies. J. Control. Release 2005, 104, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Karydas, A.G.; Ithakissios, D.S. PLGA-mPEG nanoparticles of cisplatin: In vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release 2002, 79, 123–135. [Google Scholar] [CrossRef]
- Meng, F.T.; Ma, G.H.; Qiu, W.; Su, Z.G. W/O/W double emulsion technique using ethyl acetate as organic solvent: Effects of its diffusion rate on the characteristics of microparticles. J. Control. Release 2003, 91, 407–416. [Google Scholar] [CrossRef]
- Song, C.X.; Labhasetwar, V.; Murphy, H.; Qu, X.; Humphrey, W.R.; Shebuski, R.J.; Levy, R.J. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Control. Release 1997, 43, 197–212. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.H. Self-assembled biodegradable nanoparticles developed by direct dialysis for the delivery of paclitaxel. Pharm. Res. 2005, 22, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Jette, K.; Law, D.; Schmitt, E.; Kwon, G. Preparation and drug loading of poly(ethylene glycol)-block-poly(ε-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharm. Res. 2004, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Pinon-Segundo, E.; Nava-Arzaluz, M.G.; Lechuga-Ballesteros, D. Pharmaceutical polymeric nanoparticles prepared by the double emulsion- solvent evaporation technique. Recent Pat. Drug Deliv. Formul. 2012, 6, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Dufresne, M.H.; Smith, D.C.; Ranger, M.; Leroux, J.C. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm. Res. 2004, 21, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; van Colen, G.; Sander, B.; Golas, M.; Uezguen, S.; Weigandt, M.; Goepferich, A. Drug Loading of Polymeric Micelles. Pharm. Res. 2013, 30, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliver. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Angammana, C.J.; Jayaram, S.H. Effects of ionic carriers on the morphological properties of electrospun nanofibres. In Electrical Insulation and Dielectric Phenomena (CEIDP). Proceedings of the 2010 Annual Report Conference, West Lafayette, IN, USA, 17–20 October 2010; IEEE: West Lafayette, IN, USA, 2010; pp. 1–4. [Google Scholar]
- Asare-Addo, K.; Conway, B.R.; Larhrib, H.; Levina, M.; Rajabi-Siahboomi, A.R.; Tetteh, J.; Boateng, J.; Nokhodchi, A. The effect of pH and ionic strength of dissolution media on in vitro release of two model drugs of different solubilities from HPMC matrices. Colloids Surf. B Biointerfaces 2013, 111, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Emileh, A.; Vasheghani-Farahani, E.; Imani, M. Swelling behavior, mechanical properties and network parameters of pH- and temperature-sensitive hydrogels of poly((2-dimethyl amino) ethyl methacrylate-co-butyl methacrylate). Eur. Polym. J. 2007, 43, 1986–1995. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Cao, S.; Tan, H.; Li, J.; Xu, F.; Zhang, X. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly(vinyl alcohol) and star poly[2-(dimethylamino)ethyl methacrylate]. Int. J. Pharm. 2011, 416, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Kang, H.; Lee, Y.; Bae, Y. pH-sensitive polymers for drug delivery. Macromol. Res. 2012, 20, 224–233. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Aguilar, M.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J. Smart Polymers and Their Applications as Biomaterials. Top. Tissue Eng. 2007, 3, 6. [Google Scholar]
- Kim, S.Y.; Cho, S.M.; Lee, Y.M.; Kim, S.J. Thermo- and pH-responsive behaviors of graft copolymer and blend based on chitosan and N-isopropylacrylamide. J. Appl. Polym. Sci. 2000, 78, 1381–1391. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Cheung, C.Y.; Murthy, N.; Bornstein, P.; Stayton, P.S.; Hoffman, A.S. pH-Sensitive polymers that enhance intracellular drug delivery in vivo. J. Control. Release 2002, 78, 295–303. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.H. Novel pH-sensitive polymers containing sulfonamide groups. Macromol. Rapid Commun. 1999, 20, 269–273. [Google Scholar] [CrossRef]
- Ghosh, P. Hydrophilic polymeric nanoparticles as drug carriers. Indian J. Biochem. Biophys. 2000, 37, 273–282. [Google Scholar]
- Nagavarma, B.; Ayaz, A.; Vasudha, L.; Shivakumar, H. Different Techniques for Preparation of Polymeric Nanoparticles—A Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Tang, W.; Xu, H.; Park, E.J.; Philbert, M.A.; Kopelman, R. Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness. Biochem. Biophys. Res. Commun. 2008, 369, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Gao, D.; Agayan, R.R.; Xu, H.; Philbert, M.A.; Kopelman, R. Nanoparticles for Two-Photon Photodynamic Therapy in Living Cells. Nano Lett. 2006, 6, 2383–2386. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bearinger, J.; Lautenschlager, E.; Castner, D.; Healy, K. Surface modification of poly (ethylene terephthalate) angioplasty balloons with a hydrophilic poly (acrylamide-co-ethylene glycol) interpenetrating polymer network coating. J. Biomed. Mater. Res. 2000, 53, 568–576. [Google Scholar] [CrossRef]
- Moreno, M.J.; Monson, E.; Reddy, R.G.; Rehemtulla, A.; Ross, B.D.; Philbert, M.; Schneider, R.J.; Kopelman, R. Production of singlet oxygen by Ru(dpp(SO3)2)3 incorporated in polyacrylamide PEBBLES. Sens. Actuators B Chem. 2003, 90, 82–89. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Bertin, A. Emergence of Polymer Stereocomplexes for Biomedical Applications. Macromol. Chem. Phys. 2012, 213, 2329–2352. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Bergsma, J.E.; Rozema, F.R.; Bos, R.R.M.; Boering, G.; de Bruijn, W.C.; Pennings, A.J. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polylactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Lavie, G.; Mazur, Y.; Lavie, D.; Meruelo, D. The chemical and biological properties of hypericin—A compound with a broad spectrum of biological activities. Med. Res. Rev. 1995, 15, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int. J. Pharm. 2006, 326, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tian, W.; Zhu, Y.; Bai, Y.; Yan, H.; Du, J. How does a tiny terminal alkynyl end group drive fully hydrophilic homopolymers to self-assemble into multicompartment vesicles and flower-like complex particles? Poly. Chem. 2014, 5, 5077–5088. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliver. Rev. 2001, 53, 95–108. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Caldorera-Moore, M.; Guimard, N.; Shi, L.; Roy, K. Designer nanoparticles: Incorporating size, shape, and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010, 7, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Pegaz, B.; Debefve, E.; Konan-Kouakou, Y.; Lange, N.; Ballini, J.-P.; van den Bergh, H.; Gurny, R.; Delie, F. Improved photodynamic activity of porphyrin loaded into nanoparticles: An in vivo evaluation using chick embryos. Int. J. Pharm. 2004, 286, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Eid, M.; Fanchaouy, M.; Gurny, R.; Delie, F. In vivo photodynamic activity of photosensitizer-loaded nanoparticles: Formulation properties, administration parameters and biological issues involved in PDT outcome. Eur. J. Pharm. Biopharm. 2008, 69, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Junior, E.; Marchetti, J.M. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 2006, 310, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG-PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Nakada, Y.; Sakurai, K.; Nakamura, T.; Takahashi, Y. Preparation of nanoparticles consisted of poly(l-lactide)–poly(ethylene glycol)–poly(l-lactide) and their evaluation in vitro. Int. J. Pharm. 1999, 185, 93–101. [Google Scholar] [CrossRef]
- Yang, Z.L.; Li, X.R.; Yang, K.W.; Liu, Y. Amphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: Preparation, freeze-drying, and in vitro release. J. Biomed. Mater. Res. Part A 2008, 85, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.M.; Ding, H.; Kessinger, C.W.; Khemtong, C.; Gao, J.; Sumer, B.D. Polymeric micelle nanoparticles for photodynamic treatment of head and neck cancer cells. Otolaryngol. Head Neck Surg. 2010, 143, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, O.; Mosqueira, V.; Legrand, P.; Blais, J. A comparative study of the cellular uptake, localization and phototoxicity of meta-tetra (hydroxyphenyl) chlorin encapsulated in surface-modified submicronic oil/water carriers in HT29 tumor cells. J. Photochem. Photobiol. B Biol. 2000, 55, 164–171. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.; Mishra, S.; Kulkarni, R. Formulation and Evaluation of Glipizide loaded nanoparticles. J. Pharm. Pharm. Sci. 2013, 5, 147–151. [Google Scholar]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly (ε-caprolactone)-poly (ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Gupta, A.S. Delivery of the photosensitizer Pc 4 in PEG-PCL micelles for in vitro PDT studies. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [PubMed]
- Peng, C.L.; Shieh, M.J.; Tsai, M.H.; Chang, C.C.; Lai, P.S. Self-assembled star-shaped chlorin-core poly(epsilon-caprolactone)-poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials 2008, 29, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Quynh, T.M.; Yoneyamab, M.; Maki, Y.; Dobashi, T. Poly(N-isopropylacrylamide-co-hydroxyethyl methacrylate) graft copolymers and their application as carriers for drug delivery system. J. Appl. Polym. Sci. 2012, 123, 2368–2376. [Google Scholar] [CrossRef]
- Shinoda, H.; Miller, P.J.; Matyjaszewski, K. Improving the Structural Control of Graft Copolymers by Combining ATRP with the Macromonomer Method. Macromolecules 2001, 34, 3186–3194. [Google Scholar] [CrossRef]
- Xun, W.; Wang, H.-Y.; Li, Z.-Y.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Self-assembled micelles of novel graft amphiphilic copolymers for drug controlled release. Colloids Surf. B Biointerfaces 2011, 85, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, O.; Laville, I.; Carrez, D.; Croisy, A.; Fedel, P.; Kasselouri, A.; Prognon, P.; Legrand, P.; Blais, J. Biodistribution of meta-tetra(hydroxyphenyl)chlorin incorporated into surface-modified nanocapsules in tumor-bearing mice. Photochem. Photobiol. Sci. 2002, 1, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Tsai, C.H.; Lin, S.Y.; Jhang, C.R.; Chiang, Y.S.; Hsiue, G.H. Stimulated release of photosensitizers from graft and diblock micelles for photodynamic therapy. Biomaterials 2012, 33, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [PubMed]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Jain, A.; Dubey, S.; Kaushik, A.; Tyagi, A. Dendrimer: A complete drug carrier. Int. J. Pharm. Sci. Drug Res. 2010, 1, 38–52. [Google Scholar]
- Pan, G.; Lemmouchi, Y.; Akala, E.O.; Bakare, O. Studies on PEGylated and drug-loaded PAMAM dendrimers. J. Bioact. Compat. Polym. 2005, 20, 113–128. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Battah, S.H.; Chee, C.-E.; Nakanishi, H.; Gerscher, S.; MacRobert, A.J.; Edwards, C. Synthesis and Biological Studies of 5-Aminolevulinic Acid-Containing Dendrimers for Photodynamic Therapy. Bioconjug. Chem. 2001, 12, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of Poly(ethylene glycol)-Attached Dendrimers Encapsulating Photosensitizers for Application to Photodynamic Therapy. Bioconjug. Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Komber, H.; Stumpe, K.; Voit, B. NMR Study of Hyperbranched Polyphenylenes from the AB2, (AB2 + AB) and (A2 + B3) Methods. Macromol. Chem. Phys. 2006, 207, 1814–1824. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Shi, Y.; Nabae, Y.; Hayakawa, T.; Kobayashi, H.; Yabushita, M.; Fukuoka, A.; Kakimoto, M.-A. Synthesis and characterization of hyperbranched aromatic poly(ether ketone)s functionalized with carboxylic acid terminal groups. Polym. J. 2014, 46, 722–727. [Google Scholar] [CrossRef]
- Li, P.; Zhou, G.; Zhu, X.; Li, G.; Yan, P.; Shen, L.; Xu, Q.; Hamblin, M.R. Photodynamic therapy with hyperbranched poly(ether-ester) chlorin(e6) nanoparticles on human tongue carcinoma CAL-27 cells. Photodiagn. Photodyn. Ther. 2012, 9, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Gupta, A.; Wang, S.; Pera, P.; Rao, K.V.R.; Patel, N.; Ohulchanskyy, T.Y.; Missert, J.; Morgan, J.; Koo-Lee, Y.-E.; Kopelman, R.; et al. Multifunctional Nanoplatforms for Fluorescence Imaging and Photodynamic Therapy Developed by Post-loading Photosensitizer and Fluorophore to Polyacrylamide Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, H.; Philbert, M.A.; Kopelman, R. Ultrafine hydrogel nanoparticles: Synthetic approach and therapeutic application in living cells. Angew. Chem. 2007, 46, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cattran, A.W.; Nguyen, T.; Nieminen, A.-L.; Xu, P. Triple-responsive expansile nanogel for tumor and mitochondria targeted photosensitizer delivery. Biomaterials 2014, 35, 9546–9553. [Google Scholar] [CrossRef] [PubMed]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design: A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Yu, J.C. Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J. Mater. Chem. B 2014, 2, 452–470. [Google Scholar] [CrossRef]
- Ojea-Jimenez, I.; Comenge, J.; Garcia-Fernandez, L.; Megson, Z.A.; Casals, E.; Puntes, V.F. Engineered inorganic nanoparticles for drug delivery applications. Curr. Drug Metab. 2013, 14, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Dey, N.S.; Rao, M. Quantum dot: Novel carrier for drug delivery. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 448–458. [Google Scholar]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [PubMed]
- Ladj, R.; Bitar, A.; Eissa, M.; Mugnier, Y.; Le Dantec, R.; Fessi, H.; Elaissari, A. Individual inorganic nanoparticles: Preparation, functionalization and in vitro biomedical diagnostic applications. J. Mater. Chem. B 2013, 1, 1381–1396. [Google Scholar] [CrossRef]
- Thienot, E.; Germain, M.; Piejos, K.; Simon, V.; Darmon, A.; Marill, J.; Borghi, E.; Levy, L.; Hochepied, J.F.; Pottier, A. One pot synthesis of new hybrid versatile nanocarrier exhibiting efficient stability in biological environment for use in photodynamic therapy. J. Photochem. Photobiol. B Biol. 2010, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Grazu, V.; Moros, M. Nanocarriers as nanomedicines: design concepts and recent advances. In Nanobiotechnology: Inorganic Nanoparticles vs Organic Nanoparticles; de la Fuente, J.M., Grazu, V., Eds.; Elsivier: New York, NY, USA, 2012; Volume 4, pp. 337–385. [Google Scholar]
- Kim, B.-S.; Park, S.W.; Hammond, P.T. Hydrogen-Bonding Layer-by-Layer-Assembled Biodegradable Polymeric Micelles as Drug Delivery Vehicles from Surfaces. ACS Nano 2008, 2, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. Available online: http://hdl.handle.net/2003/33542 (accessed on 31 August 2015).
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Enhanced Drug Loading on Magnetic Nanoparticles by Layer-by-Layer Assembly Using Drug Conjugates: Blood Compatibility Evaluation and Targeted Drug Delivery in Cancer Cells. Langmuir 2011, 27, 14489–14496. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Targeted Intracellular Delivery of Hydrophobic Agents using Mesoporous Hybrid Silica Nanoparticles as Carrier Systems. Nano Lett. 2009, 9, 3308–3311. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design strategies and applications of circulating cell mediated drug delivery systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.; Burda, C. Nanoparticle mediated non-covalent drug delivery(). Adv. Drug Deliv. Rev. 2013, 65, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Neeleshwar, S.; Chen, C.; Tsai, C.; Chen, Y.; Chen, C.-C.; Shyu, S.; Seehra, M. Size-dependent properties of CdSe quantum dots. Phys. Rev. B 2005, 71, 201307. [Google Scholar] [CrossRef]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Zrazhevskiy, P.; Sena, M.; Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.D.; Stone, V. Quantum Dots: An Insight and Perspective of Their Biological Interaction and How This Relates to Their Relevance for Clinical Use. Theranostics 2012, 2, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Poderys, V.; Matulionyte, M.; Selskis, A.; Rotomskis, R. Interaction of water-soluble CdTe quantum dots with bovine serum albumin. Nanoscale Res. Lett. 2011, 6, 9–14. [Google Scholar] [CrossRef]
- Juzenas, P.; Chen, W.; Sun, Y.P.; Coelho, M.A.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Gao, Y.; Yan, F. Semiconductor Quantum Dots for Biomedicial Applications. Sensors 2011, 11, 11736–11751. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.; Poznyak, S.; Gaponik, N.; Rogach, A.; Eychmüller, A. Synthesis of surface-modified colloidal semiconductor nanocrystals and study of photoinduced charge separation and transport in nanocrystal-polymer composites. Phys. E: Low-Dimens. Syst. Nanostruct. 2002, 14, 237–241. [Google Scholar] [CrossRef]
- Samia, A.C.S.; Chen, X.; Burda, C. Semiconductor Quantum Dots for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.B.; Ghaderi, S.; Keshtgar, M.; Seifalian, A.M. Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev. 2010, 1, 10.3402. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet Oxygen Production by Peptide-Coated Quantum Dot−Photosensitizer Conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—The trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149. [Google Scholar] [CrossRef]
- Moreno-Vega, A.-I.; Gómez-Quintero, T.; Nuñez-Anita, R.-E.; Acosta-Torres, L.-S.; Castaño, V. Polymeric and Ceramic Nanoparticles in Biomedical Applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.; Sehgal, R.; Hietala, S.; Deshpande, R.; Smith, D.; Loy, D.; Ashley, C. Sol-gel strategies for controlled porosity inorganic materials. J. Membr. Sci. 1994, 94, 85–102. [Google Scholar] [CrossRef]
- Corriu, R.J. Ceramics and nanostructures from molecular precursors. Angew. Chem. Int. Ed. 2000, 39, 1376–1398. [Google Scholar] [CrossRef]
- Lin, Y.; Kumakiri, I.; Nair, B.; Alsyouri, H. Microporous inorganic membranes. Sep. Purif. Rev. 2002, 31, 229–379. [Google Scholar] [CrossRef]
- Yang, L.; Sheldon, B.W.; Webster, T.J. Nanophase ceramics for improved drug delivery. Am. Ceram. Soc. Bull. 2010, 89, 24–32. [Google Scholar]
- Yang, G.; Gong, H.; Qian, X.; Tan, P.; Li, Z.; Liu, T.; Liu, J.; Li, Y.; Liu, Z. Mesoporous silica nanorods intrinsically doped with photosensitizers as a multifunctional drug carrier for combination therapy of cancer. Nano Res. 2014, 3, 751–764. [Google Scholar] [CrossRef]
- Kossovsky, N.; Gelman, A.; Sponsler, E.E.; Hnatyszyn, H.J.; Rajguru, S.; Torres, M.; Pham, M.; Crowder, J.; Zemanovich, J.; Chung, A. Surface-modified nanocrystalline ceramics for drug delivery applications. Biomaterials 1994, 15, 1201–1207. [Google Scholar] [CrossRef]
- Pandey, R.S.; Sahu, S.; Sudheesh, M.; Madan, J.; Kumar, M.; Dixit, V.K. Carbohydrate modified ultrafine ceramic nanoparticles for allergen immunotherapy. Int. Immunopharmacol. 2011, 11, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-Based Nanoparticles Entrapping Water-Insoluble Photosensitizing Anticancer Drugs: A Novel Drug−Carrier System for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Teng, I.T.; Chang, Y.J.; Wang, L.S.; Lu, H.Y.; Wu, L.C.; Yang, C.M.; Chiu, C.C.; Yang, C.H.; Hsu, S.L.; Ho, J.A. Phospholipid-functionalized mesoporous silica nanocarriers for selective photodynamic therapy of cancer. Biomaterials 2013, 34, 7462–7470. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhou, L.; Lin, Y.; Wang, A.; Zhou, J.H.; Wei, S.H. Property study of a new silica nanoparticle delivery system of hydrophobic phthalocyanine using spectroscopic method. Spectroscopy 2011, 26, 179–185. [Google Scholar] [CrossRef]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morere, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 12, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Brevet, D.; Benkirane-Jessel, N.; Raehm, L.; Maillard, P.; Garcia, M.; Durand, J.O. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagn. Photodyn. Ther. 2012, 9, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; et al. Mannose-Functionalized Mesoporous Silica Nanoparticles for Efficient Two-Photon Photodynamic Therapy of Solid Tumors. Angew. Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 751075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kim, T.-H.; Ahn, J.-C.; Kim, H.-W.; Kim, S.Y. Highly efficient “theranostics” system based on surface-modified gold nanocarriers for imaging and photodynamic therapy of cancer. J. Mater. Chem. B 2013, 42, 5806–5817. [Google Scholar] [CrossRef]
- Bauer, B.; Chen, S.; Käll, M.; Gunnarsson, L.; Ericson, M.B. Optical Interactions with Tissue and Cells XXII. In Metal Nanoparticles Amplify Photodynamic Effect on Skin Cells in Vitro. Proceedings of the SPIE, San Francisco, CA, USA, 22 January 2011; Jansen, E.D., Thomas, R.J., Eds.; p. 789712.
- Cheng, Y.; Doane, T.L.; Chuang, C.H.; Ziady, A.; Burda, C. Near infrared light-triggered drug generation and release from gold nanoparticle carriers for photodynamic therapy. Small 2014, 10, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.S.; Chang, Y.T.; Cho, K.C.; Chiu, K.C.; Lien, C.H.; Yeh, C.S.; Chen, S.J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, G.; You, M.; Song, E.; Shukoor, M.I.; Zhang, K.; Altman, M.B.; Chen, Y.; Zhu, Z.; Huang, C.Z.; et al. Assembly of Aptamer Switch Probes and Photosensitizer on Gold Nanorods for Targeted Photothermal and Photodynamic Cancer Therapy. ACS Nano 2012, 6, 5070–5077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.H.; et al. Gold nanorod enhanced two-photon excitation fluorescence of photosensitizers for two-photon imaging and photodynamic therapy. Acs Appl. Mater. Int. 2014, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Chouikrat, R.; Seve, A.; Vanderesse, R.; Benachour, H.; Barberi-Heyob, M.; Richeter, S.; Raehm, L.; Durand, J.O.; Verelst, M.; Frochot, C. Non polymeric nanoparticles for photodynamic therapy applications: Recent developments. Curr. Med. Chem. 2012, 19, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerald, S.; Beeby, A.; Chambrier, I.; Cook, M.J.; Russell, D.A. Generation of Cytotoxic Singlet Oxygen via Phthalocyanine-Stabilized Gold Nanoparticles: A Potential Delivery Vehicle for Photodynamic Therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar] [CrossRef]
- Wieder, M.E.; Hone, D.C.; Cook, M.J.; Handsley, M.M.; Gavrilovic, J.; Russell, D.A. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: Cancer therapy using a “Trojan horse”. Photochem. Photobiol. Sci. 2006, 5, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ohta, S.; Sonoda, A.; Yamada, M.; Yamamoto, M.; Nitta, N.; Murata, K.; Tabata, Y. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J. Control. Release 2007, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: Carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar]
- Wang, S.; Gao, R.; Zhou, F.; Selke, M. Nanomaterials and singlet oxygen photosensitizers: Potential applications in photodynamic therapy. J. Mater. Chem. 2004, 14, 487. [Google Scholar] [CrossRef]
- Huang, L.; Terakawa, M.; Zhiyentayev, T.; Huang, Y.Y.; Sawayama, Y.; Jahnke, A.; Tegos, G.P.; Wharton, T.; Hamblin, M.R. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Pawlak, A.; Satti, M.; Lee, H.; Wharton, T.; Gali, H.; Sarna, T.; Hamblin, M.R. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic. Biol. Med. 2007, 43, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Todorovic-Markovic, B.; Kleut, D.; Nikolic, N.; Vranjes-Djuric, S.; Misirkic, M.; Vucicevic, L.; Janjetovic, K.; Isakovic, A.; Harhaji, L.; et al. The mechanism of cell-damaging reactive oxygen generation by colloidal fullerenes. Biomaterials 2007, 28, 5437–5448. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−* versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Erbas, S.; Gorgulu, A.; Kocakusakogullari, M.; Akkaya, E.U. Non-covalent functionalized SWNTs as delivery agents for novel Bodipy-based potential PDT sensitizers. Chem.Commun. 2009, 33, 4956–4958. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qi, Y.; Liu, B. Polyacrylic Acid Functionalized Nanographene as a Nanocarrier for Loading and Controlled Release of Doxorubicin Hydrochloride. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, T.; Wang, H.; Gu, Y.; Xu, Y.; Tang, H.; Jia, G.; Liu, Y. Biocompatibility of graphene oxide intravenously administrated in mice—effects of dose, size and exposure protocols. Toxicol. Res. 2015, 4, 83–91. [Google Scholar] [CrossRef]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Herman, M.; Kessel, D.; Fromm, D. Current Concepts in Gastrointestinal Photodynamic Therapy. An. Surg. 1999, 230, 12. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Wulf, H.C.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.J.P.; Gilaberte, Y.; Calzavara-Pinton, P.; Morton, C.A.; Sidoroff, A.; et al. Daylight photodynamic therapy for actinic keratosis: An international consensus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Smith, K.M.; McCullough, J.L.; Berns, M.W. Skin photosensitivity and photodestruction of several potential photodynamic sensitizers. Photochem. Photobiol. 1989, 49, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.B.; Wennberg, A.-M.; Larkö, O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther. Clin. Risk Manag. 2008, 4, 1–9. [Google Scholar] [PubMed]
- Kapur, N.; Lang, K.K.; Braathen, L. Photodynamic therapy-induced pain: A patient centred survey. J. Am. Acad. Dermatol. 2004, 50, 136. [Google Scholar] [CrossRef]
- Kasche, A.; Luderschmidt, S.; Ring, J.; Hein, R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drug. Dermatol. JDD 2006, 5, 353–356. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debele, T.A.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094-22136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922094
Debele TA, Peng S, Tsai H-C. Drug Carrier for Photodynamic Cancer Therapy. International Journal of Molecular Sciences. 2015; 16(9):22094-22136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922094
Chicago/Turabian StyleDebele, Tilahun Ayane, Sydney Peng, and Hsieh-Chih Tsai. 2015. "Drug Carrier for Photodynamic Cancer Therapy" International Journal of Molecular Sciences 16, no. 9: 22094-22136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922094







