MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line
Abstract
:1. Introduction
2. Results and Discussion
2.1. MiR-122 Is Detectable in Hepatocyte Cell Lines but Not NSCLC Cells Lines

2.2. MiR-122 Suppresses Proliferation of NSCLC A549 Cells
2.3. MiR-122 Enhances the Radiosensitization of A549 Cells

2.4. MiR-122 Enhances the DNA Double Strand Break (DSB) and Apoptosis Induced by IR

2.5. MiR-122 Enhances the A549 Cells Anchorage-Independent Growth Inhibition Induced by IR

2.7. MiR-122 Reduces the Expression of Its Pro-Survival or Anti-Apoptosis Targeted Genes in A549 Cells

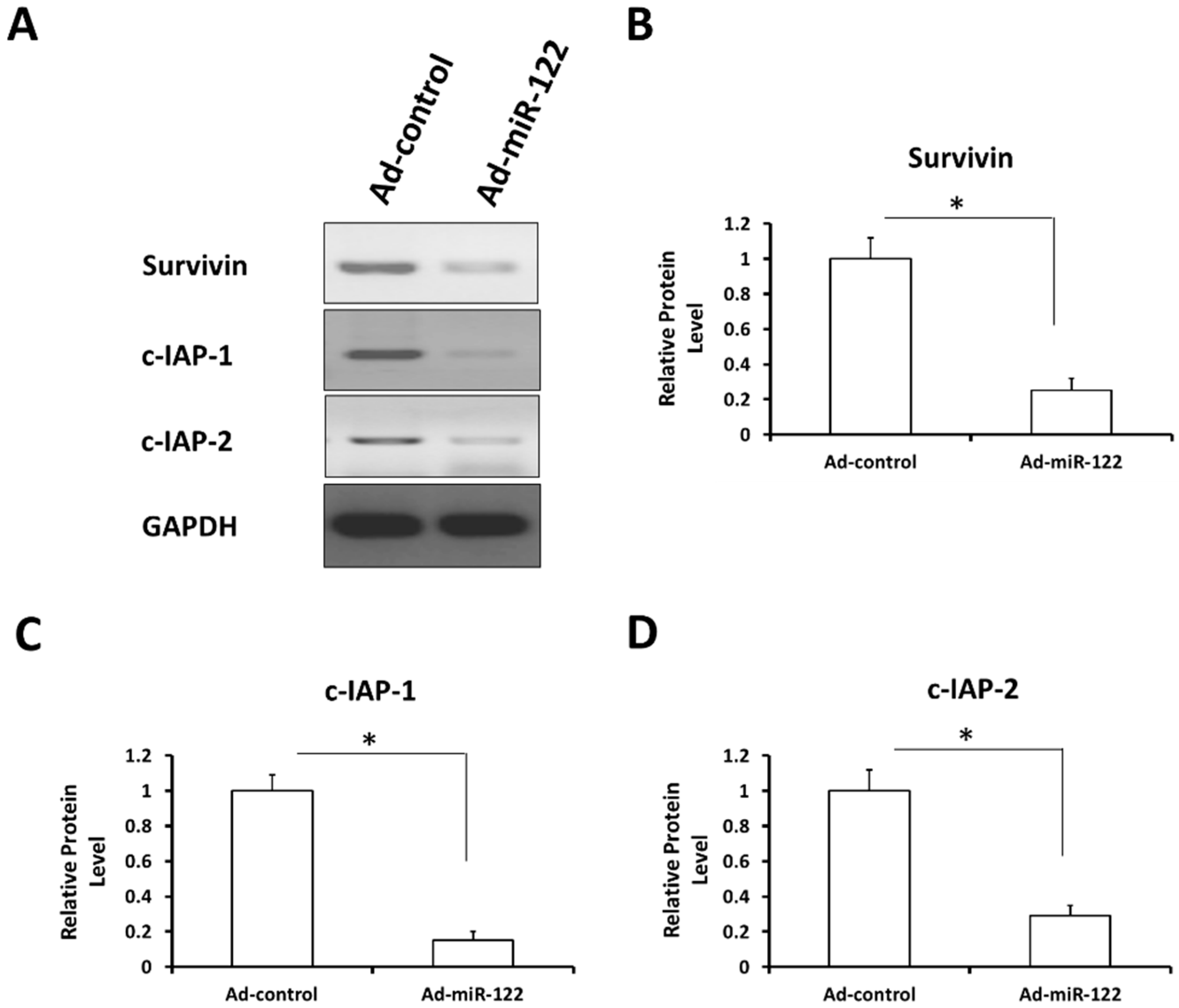
2.8. MiR-122 Reduces the Expression of Stress-Response Regulators Survivin, c-IAP-1 and c-IAP-2 in A549 Cells
3. Experimental Section
3.1. Cell Culture and Proliferation Analysis
3.2. Adenovirus Vector Preparation
3.3. RNA Isolation and Real-Time Quantitative RT-PCR (Real Time qRT-PCR)
3.4. Colony Formation
3.5. Immunocytochemistry (Cellular Immunofluorescence)
3.6. Apoptosis and Flow Cytometry Analysis
3.7. Anchorage-Independent Growth
3.8. Tranwell Invasion Assay
3.9. Antibodies and Western Blot
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bussink, J.; van der Kogel, A.J.; Kaanders, J.H. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008, 9, 288–296. [Google Scholar] [CrossRef]
- Niu, C.; Liang, C.; Guo, J.; Cheng, L.; Zhang, H.; Qin, X.; Zhang, Q.; Ding, L.; Yuan, B.; Xu, X.; et al. Downregulation and growth inhibitory role of FHL1 in lung cancer. Int. J. Cancer 2012, 130, 2549–2956. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.D.; Simon, M.C. Novel insights into the molecular origins and treatment of lung cancer. Cell Cycle 2010, 9, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Eliasz, S.; Liang, S.; Chen, Y.; de Marco, M.A.; Machek, O.; Skucha, S.; Miele, L.; Bocchetta, M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 2010, 29, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Viral and cellular messenger RNA targets of viral microRNAs. Nature 2009, 457, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Nag, S.A.; Su, Y.; Voruganti, S.; Qin, J.J.; Zhang, R.; Cho, W.C. miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents. Curr. Cancer Drug Targets 2013, 13, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Shen, J.; Liu, L.; Wu, J.; Li, W.; Luo, J.; Chen, Q.; Qian, C. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol. Ther. 2010, 9, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.B.; Feng, F.; Zhang, F.; Yang, J.L.; Shi, G.B.; Han, Y.L.; Zhang, Z.Y. MicroRNA122 enhances the cytotoxic activity of Gemcitabine on A549 cells. Acad. J. Chin. PLA Med. Sch. 2014, 35, 1160–1164. [Google Scholar]
- Kutay, H.; Bai, S.; Datta, J.; Motiwala, T.; Pogribny, I.; Frankel, W.; Jacob, S.T.; Ghoshal, K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J. Cell. Biochem. 2006, 99, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Nasser, M.W.; Wang, B.; Hsu, S.H.; Datta, J.; Kutay, H.; Yadav, A.; Nuovo, G.; Kumar, P.; Ghoshal, K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 2009, 284, 32015–32027. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.; Gramantieri, L.; Giovannini, C.; Veronese, A.; Ferracin, M.; Sabbioni, S.; Calin, G.A.; Grazi, G.L.; Croce, C.M.; Tavolari, S.; et al. miR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009, 69, 5761–5767. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, S.H.; Yang, J.L. Research of specific of properties of Survivin and anti-tumor drugs. J. Chin. PLA Postgrad. Med. Sch. 2009, 30, 755–757. [Google Scholar]
- Chen, Y.; Feng, F.; Gao, X.; Wang, C.; Sun, H.; Zhang, C.; Zeng, Z.; Lu, Y.; An, L.; Qu, J.; et al. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells. Curr. Cancer Drug Targets 2015, 15, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Y.; Deeb, D.; Arbab, A.S.; Gautam, S.C. Anticancer activity of pristimerin in ovarian carcinoma cells is mediated through the inhibition of prosurvival Akt/NF-κB/mTOR signaling. J. Exp. Ther. Oncol. 2014, 10, 275–283. [Google Scholar] [PubMed]
- Feng, Y.; Xu, X.; Zhang, Y.; Ding, J.; Wang, Y.; Zhang, X.; Wu, Z.; Kang, L.; Liang, Y.; Zhou, L.; et al. HPIP is upregulated in colorectal cancer and regulates colorectal cancer cell proliferation, apoptosis and invasion. Sci. Rep. 2015, 5, 9429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Qu, J.H.; Chang, X.J.; Lu, Y.Y.; Bai, W.L.; Dong, Z.; Wang, H.; An, L.J.; Xu, Z.X.; Wang, C.P. High intratumoral metastasis-associated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma. J. Transl. Med. 2013, 11, 41. [Google Scholar] [CrossRef]
- Tsai, P.C.; Bake, S.; Balaraman, S.; Rawlings, J.; Holgate, R.R.; Dubois, D.; Miranda, R.C. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biol. Open 2014, 3, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Youn, H.; Kwon, T.; Kang, J.; Kim, E.; Son, B.; Yang, H.J.; Jung, Y.; Youn, B. PIM1 kinase inhibitors induce radiosensitization in non-small cell lung cancer cells. Pharmacol. Res. 2013, 70, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, M.; Feng, F.; Yang, Y.; Hang, X.; Cui, J.; Gao, J. MEIS1 functions as a potential AR negative regulator. Exp. Cell Res. 2014, 328, 58–68. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, X.; Song, E.; Chen, W.; Pang, H.; Ni, D.; Gao, Y.; Fan, Y.; Ding, Q.; Zhang, Y.; et al. Tubulin cofactor A functions as a novel positive regulator of ccRCC progression, invasion and metastasis. Int. J. Cancer 2013, 133, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feng, F.; Yang, Y.; Gao, X.; Cui, J.; Zhang, C.; Zhang, F.; Xu, Z.; Qv, J.; Wang, C.; et al. LINE-1 ORF-1p functions as a novel androgen receptor co-activator and promotes the growth of human prostatic carcinoma cells. Cell Signal. 2013, 25, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, M.; Zhang, F.; Feng, F.; Chen, W.; Yang, Y.; Cui, J.; Zhang, D.; Linghu, E. FBI-1 enhances ETS-1 signaling activity and promotes proliferation of human colorectal carcinoma cells. PLoS ONE 2014, 9, e98041. [Google Scholar] [CrossRef] [PubMed]
- Egloff, A.M.; Rothstein, M.E.; Seethala, R.; Siegfried, J.M.; Grandis, J.R.; Stabile, L.P. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 6529–6540. [Google Scholar] [CrossRef]
- Yang, Q.; Feng, F.; Zhang, F.; Wang, C.; Lu, Y.; Gao, X.; Zhu, Y.; Yang, Y. LINE-1 ORF-1p functions as a novel HGF/ETS-1 signaling pathway co-activator and promotes the growth of MDA-MB-231 cell. Cell Signal. 2013, 25, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Jia, H.; Qin, M.; Dai, W.; Wang, T.; Liang, E.; Dong, G.; Wang, Z.; Zhang, Z.; Feng, F. MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line. Int. J. Mol. Sci. 2015, 16, 22137-22150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922137
Ma D, Jia H, Qin M, Dai W, Wang T, Liang E, Dong G, Wang Z, Zhang Z, Feng F. MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line. International Journal of Molecular Sciences. 2015; 16(9):22137-22150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922137
Chicago/Turabian StyleMa, Debin, Hui Jia, Mengmeng Qin, Wenjie Dai, Tao Wang, Erguang Liang, Guofu Dong, Zuojun Wang, Zhiyuan Zhang, and Fan Feng. 2015. "MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line" International Journal of Molecular Sciences 16, no. 9: 22137-22150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160922137





