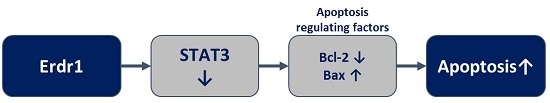
Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Recombinant Murine Erdr1 Inhibits Tumor Growth in Vivo

2.2. Erdr1 Induces Apoptosis via Regulation of Bcl-2 and Bax in Vivo

2.3. Erdr1 Induces Apoptosis in Murine Melanoma Cells, B16F10 in Vitro

3. Discussion
4. Materials and Methods
4.1. In Vivo Tumorigenecity Model
4.2. Analysis of Apoptosis by Flow Cytometry
4.3. TUNNEL Assay
4.4. Western Blot Analysis
4.5. Immunohistochemistry Analyses
4.6. Cell Culture
4.7. Measurement of STAT3 Activity
4.8. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Bowman, T.; Huang, M.; Shivers, S.; Reintgen, D.; Daud, A.; Chang, A.; Kraker, A.; Jove, R.; Yu, H. Roles of activated SRC and STAT3 signaling in melanoma tumor cell growth. Oncogene 2002, 21, 7001–7010. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.M.; Boyd, S.C.; Mijatov, B.; Gowrishankar, K.; Snoyman, S.; Pupo, G.M.; Scolyer, R.A.; Mann, G.J.; Kefford, R.F.; Zhang, X.D.; et al. Mutant B-RAF-Mcl-1 survival signaling depends on the STAT3 transcription factor. Oncogene 2014, 33, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Dormer, P.; Spitzer, E.; Frankenberger, M.; Kremmer, E. Erythroid differentiation regulator (EDR), a novel, highly conserved factor I. Induction of haemoglobin synthesis in erythroleukaemic cells. Cytokine 2004, 26, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Park, Y.; Song, S.B.; Cheon, S.Y.; Park, S.; Houh, Y.; Ha, S.; Kim, H.J.; Park, J.M.; Kim, T.S.; et al. Erythroid differentiation regulator 1, an interleukin 18-regulated gene, acts as a metastasis suppressor in melanoma. J. Investig. Dermatol. 2011, 131, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Houh, Y.K.; Ha, S.; Yang, Y.; Kim, D.; Kim, T.S.; Yoon, S.R.; Bang, S.I.; Cho, B.J.; Lee, W.J.; et al. Recombinant Erdr1 suppresses the migration and invasion ability of human gastric cancer cells, SNU-216, through the JNK pathway. Immunol. Lett. 2013, 150, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Huh, S.Y.; Hur, D.Y.; Jeong, H.; Kim, T.S.; Kim, S.Y.; Park, S.B.; Yang, Y.; Bang, S.I.; Park, H.; et al. ERDR1 enhances human NK cell cytotoxicity through an actin-regulated degranulation-dependent pathway. Cell. Immunol. 2014, 292, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.T.; Mendoza, L.; Valcarcel, M.; Salado, C.; Egilegor, E.; Telleria, N.; Vidal-Vanaclocha, F.; Dinarello, C.A. Interleukin-18 binding protein reduces B16 melanoma hepatic metastasis by neutralizing adhesiveness and growth factors of sinusoidal endothelium. Cancer Res. 2003, 63, 491–497. [Google Scholar] [PubMed]
- Kim, H.J.; Song, S.B.; Yang, Y.; Eun, Y.S.; Cho, B.K.; Park, H.J.; Cho, D.H. Erythroid differentiation regulator 1 (Erdr1) is a proapototic factor in human keratinocytes. Exp. Dermatol. 2011, 20, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.S.; Cai, J.M.; Ni, J.; Xiang, Y.S.; Cui, J.G.; Zhu, D.; Dong, J.R. Effects of STAT3 antisense oligodeoxynucleotides on apoptosis and proliferation of mouse melanoma cell line B16. Chin. J. Cancer 2006, 25, 269–274. (In Chinese) [Google Scholar]
- Bill, M.A.; Fuchs, J.R.; Li, C.; Yui, J.; Bakan, C.; Benson, D.M., Jr.; Schwartz, E.B.; Abdelhamid, D.; Lin, J.; Hoyt, D.G.; et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Bektas, M.; Wieder, T.; Daniel, P.; Eberle, J.; Orfanos, C.E.; Geilen, C.C. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS Lett. 2000, 473, 27–32. [Google Scholar] [CrossRef]
- Helmbach, H.; Rossmann, E.; Kern, M.A.; Schadendorf, D. Drug-resistance in human melanoma. Int. J. Cancer 2001, 93, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.P.; Cao, X. Regulation of STAT3 activation by MEK kinase 1. J. Biol. Chem. 2001, 276, 21004–21011. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hutzen, B.; Li, P.K.; Ball, S.; Zuo, M.; DeAngelis, S.; Foust, E.; Sobo, M.; Friedman, L.; Bhasin, D.; et al. A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells. Neoplasia 2010, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Real, P.J.; Sierra, A.; de Juan, A.; Segovia, J.C.; Lopez-Vega, J.M.; Fernandez-Luna, J.L. Resistance to chemotherapy via STAT3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene 2002, 21, 7611–7618. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Reuland, S.N.; Goldstein, N.B.; Partyka, K.A.; Smith, S.; Luo, Y.; Fujita, M.; Gonzalez, R.; Lewis, K.; Norris, D.A.; Shellman, Y.G. ABT-737 synergizes with Bortezomib to kill melanoma cells. Biol. Open 2012, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jung, M.K.; Park, H.J.; Kim, K.E.; Cho, D. Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis. Int. J. Mol. Sci. 2016, 17, 107. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010107
Lee J, Jung MK, Park HJ, Kim KE, Cho D. Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis. International Journal of Molecular Sciences. 2016; 17(1):107. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010107
Chicago/Turabian StyleLee, Joohyun, Min Kyung Jung, Hyun Jeong Park, Kyung Eun Kim, and Daeho Cho. 2016. "Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis" International Journal of Molecular Sciences 17, no. 1: 107. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010107