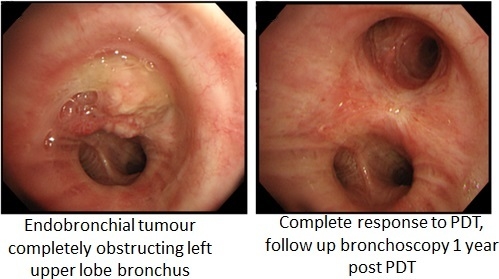
Photodynamic Therapy in Non-Gastrointestinal Thoracic Malignancies
Abstract
:1. Introduction
2. Non-Small Cell Lung Cancer
2.1. Early Cancers
2.1.2. Radiographically Occult Lung Cancer

2.2.2. Early Non-Radiographically Occult Lung Cancer (ROLC) Cancers
2.2. Advanced Lung Cancer
| Author, Publication Year | Study Type, n | Effect of Intervention | Median Follow-up Period (Range) | Complications |
|---|---|---|---|---|
| ROLC | ||||
| Noordegraaf, 2003 [15] | Case series, 32 (5 patients received BT + PDT) | 5 y OS: 50% | 5.3 years (2–11) | Local recurrence, pulmonary fibrosis, emphysema, metastasis, death |
| Endo, 2009 [17] | Case series, 48 | 5 y OS: 81%; 10 y OS: 71% | 5.25 years (1–12) | Local recurrence, second primary lung cancer, death |
| Cortese, 1997 [18] | Case series, 21 | 5 y OS: 72% | 5.7 years (2–9.7) | Local recurrence, second primary lung cancer, death |
| Imamura, 1994 [19] | Case series, 29 | 5 y OS: 56% | 4 years (0.4–6.3) | Local recurrence, pyothorax, pulmonary hypertension, respiratory insufficiency, second primary lung cancer, death |
| Kato, 1993 [20] | Case series, 58 | CR: 82.8% | Not reported | Skin photosensitivity |
| Furuse, 1993 [24] | Phase II study, 54 | CR: 85% | Not reported | Elevation of ALT, pulmonary toxicity, allergic reaction, sunburn |
| Ono, 1992 [26] | Case series, 36 | 5 y OS: 43.4% | 3.75 years (1–6) | Acute leukemia, photosensitivity, excessive airway secretion, local recurrence |
| Kato, 2003 [27] | Phase II study, 41 | CR: 83%; OS not reported | Not reported | Increased CRP and sputum, cough, fever, neutropenia, leukocytosis, photosensitivity |
| Patelli, 1999 [28] | Case series, 23 | CR: 62% | Not reported | Photosensitivity |
| Edell, 1992 [29] | Case series, 13 | CR: 71% | 1.8 years (0.6–4.1) | Increased blood-tinged sputum, sunburns |
| Non-ROLC | ||||
| Balchum, 1984 [30] | Case series, 22 | CR: 91% | Not reported | Not reported |
| Li, 1984 [31] | Case series, 21 | CR: 12.5% | At least 0.3 years | No major complications reported |
| Miyazu, 2002 [32] | Case series, 12 | CR: 75% | 2.3 years (1–3.5) | Not reported |
| Kato, 1997 [33] | Case series, 26 | CR: 82.6% | Not reported | Mild skin photosensitivity |
| Kato, 1996 [34] | Case series, 240 | CR: 39.6% | Not reported | No major complications |
| Furukawa, 2005 [36] | Case series, 93 | 5 y OS: 57.9% | 0.1–5 years | Not reported |
| Mccaughan, 1986 [37] | Case series, 18 | CR: 40% | Not reported | Local recurrence, second primary lung cancer |
| Kato, 1997 [38] | Case series, 95 | CR: 81% | 0.2–16.3 years | Photosensitization |
| Lam, 1987 [40] | RCT, 11 | CR: 40% | 0.3–1 years | Excess sputum, hemoptysis, dysphagia, nausea, pruritus, hypercalcemia |
| Furukawa, 1999 [41] | Case series, 78 | CR: 75% | Not reported | Pneumonia, fever, skin sensitivity |
| Santos, 2004 [42] | Case series, 75 | 3 y OS: 50% | 0.6–1.5 years | No major complications |
| Diaz-Jiménez, 1999 [2] | RCT, 31 | CR: 7% | Not reported | Bronchitis, photosensitization, cough, death |
| LoCicero, 1990 [43] | Case series, 10 | 1 y OS: 30% | Not reported | Sunburn, mild anasarca |
| Moghissi, 1999 [44] | Case series, 100 | 2 y OS: 19% | 1–6 years | Redness of face, mild edema, sunburn |
| Hugh-Jones, 1987 [45] | Case series, 15 | CR: 30% | Not reported | Infection, breathing obstruction |
| Vincent, 1984 [46] | Case series, 17 | CR: 12% | Not reported | Excess secretions, fever, pneumonia, endotracheal candidiasis |
2.3. Miscellaneous Used in Non-Small Cell Lung Cancer (NSCLC)
2.3.1. Photodynamic Therapy (PDT) in Downsizing to Allow Resection

2.3.2. Salvage Therapy for Recurrences

2.4. Methodologic Issues
3. Pleural Malignancies
3.1. Malignant Pleural Mesothelioma
3.2. Pleural Metastases
| Author, Publication Year | Study Type, n | Effect of Intervention | Median Follow-up Period (Range) | Complications |
|---|---|---|---|---|
| Malignant Pleural Mesothelioma | ||||
| Pass, 1997 [56] | Phase III trial, 63 | Median survival: 1.2 y | Not reported | Bronchopleural fistula |
| Du, 2010 [60] | Case series, 11 | Not reported | Not reported | Radiation pneumonitis |
| Friedberg, 2003 [63] | Phase I trial, 26 | MTD: 0.1 mg/kg of Foscan PDT | Not reported | Capillary leak syndrome, wound burns, photosensitivity |
| Moskal, 1998 [64] | Case series, 40 | 2 y OS: 23% | Not reported | A-fib, sepsis, bronchopleural fistula, empyema |
| Baas, 1997 [65] | Case series, 5 | CR: 80% | 8-11 months | Metastasis, skin sensitivity |
| Friedberg, 2012 [67] | Retrospective cohort, 38 | CR: 97% | 2.9 years | Stroke, transient respiratory insufficiency, a-fib, chyle leak |
| Schouwink, 2001 [70] | Phase I/II, 28 | CR: 50%, MTD: 0.15 mg/kg | 2.6 years (0.8–4.4) | A-fib, diaphragm rupture, empyema, depression, mucus impaction, death |
| Luketich, 1996 [71] | Case report, 1 | Not reported | Not reported | Esophagopleural fistula |
| Pleural Metastases | ||||
| Chen, 2015 [77] | Case series, 18 | 5y OS: 57.4% | 3.2 years | Acute respiratory distress syndrome, air leakage, skin redness |
| Friedberg, 2004 [78] | Phase II, 22 | CR: 73.3%; 1 y OS: 68% | 2.8 years | Transaminitis, edema, transient thrombocytopenia, fever |
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| PDT | photodynamic therapy |
| PS | photosensitizer |
| NSCLC | non-small cell lung cancer |
| ROLC | radiographically occult lung cancer |
| MPM | malignant pleural mesothelioma |
References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Jimenez, J.P.; Martinez-Ballarin, J.E.; Llunell, A.; Farrero, E.; Rodríguez, A.; Castro, M.J. Efficacy and safety of photodynamic therapy vs. Nd-YAG laser resection in, NSCLC with airway obstruction. Eur. Respir. J. 1999, 14, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Chiaviello, A.; Postiglione, I.; Palumbo, G. Targets and mechanisms of photodynamic therapy in lung cancer cells: A brief overview. Cancers Basel 2011, 3, 1014–1041. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B.; Friedberg, J.S.; Glatstein, E.; Stevenson, J.P.; Sterman, D.H.; Hahn, S.M.; Cengel, K.A. Photodynamic therapy for the treatment of non-small cell lung cancer. J. Thorac. Dis. 2012, 4, 63–75. [Google Scholar] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy in oncology. Expert Opin. Pharmacother. 2001, 2, 917–927. [Google Scholar] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Rodriguez, E.M.; Naccache, R.; Forgione, P.; Lamoureux, G.; Sanz-Rodriguez, F.; Scheglmann, D.; Capobianco, J.A. Chemical modification of temoporfin—A second generation photosensitizer activated using upconverting nanoparticles for singlet oxygen generation. Chem. Commun. Camb. 2014, 50, 12150–12153. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Foster, T.H. Photophysical parameters, photosensitizer retention and tissue optical properties completely account for the higher photodynamic efficacy of meso-tetra-hydroxyphenyl-chlorin vs. Photofrin. Photochem. Photobiol. 2005, 81, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Bombelli, C.; Mannino, S.; Stringaro, A.; Toccacieli, L.; Calcabrini, A.; Colone, M.; Mangiola, A.; Maira, G.; Luciani, P. m-THPC-mediated photodynamic therapy of malignant gliomas: Assessment of a new transfection strategy. Int. J. Cancer 2007, 121, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A. Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005, 81, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Ruevekamp, M.; Out, R.; van Berkel, T.J.; Schellens, J.; Baas, P.; Stewart, F.A. The pharmacokinetic behavior of the photosensitizer meso-tetra-hydroxyphenyl-chlorin in mice and men. Cancer Chemother. Pharmacol. 2007, 60, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bromley, E.; Xu, L.; Chen, J.C.; Keltner, L. Talaporfin sodium. Expert Opin. Pharmacother. 2010, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bromley, E.; Briggs, B.; Keltner, L.; Wang, S.S. Characterization of cutaneous photosensitivity in healthy volunteers receiving talaporfin sodium. Photodermatol. Photoimmunol. Photomed. 2011, 27, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Kadambi, A.; Hill, J.S.; Waters, C.A.; Robinson, B.C.; Walker, J.P.; Fukumura, D.; Jain, R.K. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 2002, 62, 2151–2156. [Google Scholar] [PubMed]
- Vonk-Noordegraaf, A.; Postmus, P.E.; Sutedja, T.G. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: A follow-up study. Lung Cancer 2003, 39, 49–53. [Google Scholar] [CrossRef]
- Sutedja, T.G.; Postmus, P.E. Photodynamic therapy in lung cancer. A review. J. Photochem. Photobiol. B 1996, 36, 199–204. [Google Scholar] [CrossRef]
- Endo, C.; Miyamoto, A.; Sakurada, A.; Aikawa, H.; Sagawa, M.; Sato, M.; Saito, Y.; Kondo, T. Results of long-term follow-up of photodynamic therapy for roentgenographically occult bronchogenic squamous cell carcinoma. Chest 2009, 136, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cortese, D.A.; Edell, E.S.; Kinsey, J.H. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin. Proc. 1997, 72, 595–602. [Google Scholar] [CrossRef]
- Imamura, S.; Kusunoki, Y.; Takifuji, N.; Kudo, S.; Matsui, K.; Masuda, N.; Takada, M.; Negoro, S.; Ryu, S.; Fukuoka, M. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer 1994, 73, 1608–1614. [Google Scholar] [CrossRef]
- Kato, H.; Horai, T.; Furuse, K.; Fukuoka, M.; Suzuki, S.; Hiki, Y.; Ito, Y.; Mimura, S.; Tenjin, Y.; Hisazumi, H.; et al. Photodynamic therapy for cancers: A clinical trial of porfimer sodium in, Japan. Jpn. J. Cancer Res. 1993, 84, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Lam, S. Photodynamic therapy of lung cancer. Semin. Oncol. 1994, 21, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.N.; Edell, E.; Sutedja, T.; Vergnon, J.M. Treatment of early stage non-small cell lung cancer. Chest 2003, 123, 176S–180S. [Google Scholar] [CrossRef] [PubMed]
- Kato, H. Photodynamic therapy for lung cancer—A review of 19 years’ experience. J. Photochem. Photobiol. B 1998, 42, 96–99. [Google Scholar] [CrossRef]
- Furuse, K.; Fukuoka, M.; Kato, H.; Kato, H.; Horai, T.; Kubota, K.; Kodama, N.; Kusunoki, Y.; Takifuji, N.; Okunaka, T.; Konaka, C.; et al. A prospective phase, II study on photodynamic therapy with photofrin, II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J. Clin. Oncol. 1993, 11, 1852–1857. [Google Scholar] [PubMed]
- Endo, C.; Sakurada, A.; Kondo, T. Early central airways lung cancer. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Ikeda, S.; Suemasu, K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer 1992, 69, 1696–1701. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase, II clinical study of photodynamic therapy using mono-l-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Patelli, M.; Lazzari Agli, L.; Poletti, V.; Falcone, F. Photodynamic laser therapy for the treatment of early-stage bronchogenic carcinoma. Monaldi Arch. Chest Dis. 1999, 54, 315–318. [Google Scholar] [PubMed]
- Edell, E.S.; Cortese, D.A. Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest 1992, 102, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Balchum, O.J.; Doiron, D.R.; Huth, G.C. HpD photodynamic therapy for obstructing lung cancer. Prog. Clin. Biol. Res. 1984, 170, 727–745. [Google Scholar] [PubMed]
- Li, J.H.; Chen, Y.P.; Zhao, S.D.; Zhang, L.T.; Song, S.Z. Application of hematoporphyrin derivative and laser-induced photodynamical reaction in the treatment of lung cancer: A preliminary report on 21 cases. Lasers Surg. Med. 1984, 4, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Miyazu, Y.; Miyazawa, T.; Kurimoto, N.; Iwamoto, Y.; Kanoh, K.; Kohno, N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am. J. Respir Crit. Care Med. 2002, 165, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Okunaka, T.; Konaka, C.; Furuse, K.; Kusunoki, Y.; Horai, T.; Takifuji, N.; Negoro, S.; Fukuoka, M.; Yaya, T.; et al. Photodynamic therapy with YAG-OPO laser for early stage lung cancer. Diagn. Ther. Endosc. 1997, 4, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Okunaka, T.; Shimatani, H. Photodynamic therapy for early stage bronchogenic carcinoma. J. Clin. Laser Med. Surg. 1996, 14, 235–238. [Google Scholar] [PubMed]
- Moghissi, K.; Dixon, K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC). Photodiagn. Photodyn. Ther. 2008, 5, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Kato, H.; Konaka, C.; Okunaka, T.; Usuda, J.; Ebihara, Y. Locally recurrent central-type early stage lung cancer <1.0 cm in diameter after complete remission by photodynamic therapy. Chest 2005, 128, 3269–3275. [Google Scholar]
- McCaughan, J.S., Jr.; Williams, T.E., Jr.; Bethel, B.H. Photodynamic therapy of endobronchial tumors. Lasers Surg. Med. 1986, 6, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kato, H. Photodynamic therapy for early stage central type of lung cancer. Mayo Clin. Proc. 1997, 72, 688–690. [Google Scholar] [CrossRef]
- Maziak, D.E.; Markman, B.R.; MacKay, J.A.; Evans, W.K. Photodynamic therapy in nonsmall cell lung cancer: A systematic review. Ann. Thorac. Surg. 2004, 77, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Kostashuk, E.C.; Coy, E.P.; Laukkanen, E.; LeRiche, J.C.; Mueller, H.A.; Szasz, I.J. A randomized comparative study of the safety and efficacy of photodynamic therapy using, Photofrin, II combined with palliative radiotherapy vs. palliative radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. Photochem. Photobiol. 1987, 46, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Okunaka, T.; Yamamoto, H.; Tsuchida, T.; Usuda, J.; Kumasaka, H.; Ishida, J.; Konaka, C.; Kato, H. Effectiveness of photodynamic therapy and Nd-YAG laser treatment for obstructed tracheobronchial, malignancies. Diagn. Ther. Endosc. 1999, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.S.; Raftopoulos, Y.; Keenan, R.J.; Halal, A.; Maley, R.H.; Landreneau, R.J. Bronchoscopic palliation of primary lung cancer: Single or multimodality therapy? Surg. Endosc. 2004, 18, 931–936. [Google Scholar] [PubMed]
- LoCicero, J.; Metzdorff, M.; Almgren, C. Photodynamic therapy in the palliation of late stage obstructing non-small cell lung cancer. Chest 1990, 98, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K.; Dixon, K.; Stringer, M.; Freeman, T.; Thorpe, A.; Brown, S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: Experience of 100 cases. Eur. J. Cardiothorac. Surg. 1999, 15, 1–6. [Google Scholar] [CrossRef]
- Hugh-Jones, P.; Gardner, W.N. Laser photodynamic therapy for inoperable bronchogenic squamous carcinoma. Q. J. Med. 1987, 64, 565–581. [Google Scholar] [PubMed]
- Vincent, R.G.; Dougherty, T.J.; Rao, U.; Boyle, D.G.; Potter, W.R. Photoradiation therapy in advanced carcinoma of the trachea and bronchus. Chest 1984, 85, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Konaka, C.; Usuda, J.; Kato, H. Preoperative photodynamic therapy for lung cancer. Nihon Geka Gakkai Zasshi 2000, 101, 486–489. [Google Scholar] [PubMed]
- Okunaka, T.; Hiyoshi, T.; Furukawa, K.; Yamamoto, H.; Tsuchida, T.; Usuda, J.; Kumasaka, H.; Ishida, J.; Konaka, C.; Kato, H. Lung cancers treated with photodynamic therapy and surgery. Diagn. Ther. Endosc. 1999, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ross, P., Jr.; Grecula, J.; Bekaii-Saab, T.; Villalona-Calero, M.; Otterson, G.; Magro, C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg. Med. 2006, 38, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yasufuku, K.; Sakairi, Y.; Shibuya, K.; Yoshida, S.; Yoshino, I. Successful treatment of lung cancer by multimodal endobronchial interventions. Respiration 2014, 88, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K.; Dixon, K.; Thorpe, J.A.; Stringer, M.; Oxtoby, C. Photodynamic therapy (PDT) in early central lung cancer: A treatment option for patients ineligible for surgical resection. Thorax 2007, 62, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Corti, L.; Toniolo, L.; Boso, C.; Colaut, F.; Fiore, D.; Muzzio, P.C.; Koukourakis, M.I.; Mazzarotto, R.; Pignataro, M.; Loreggian, L.; et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg. Med. 2007, 39, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Briel, M.; You, J.J.; Sun, X.; Johnston, B.C.; Busse, J.W.; Mulla, S.; Lamontagne, F.; Bassler, D.; Vera, C.; et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): Systematic review. BMJ 2012, 344, e2809. [Google Scholar] [CrossRef] [PubMed]
- Kidane, B.; Plourde, M.; Chadi, S.A.; Iansavitchene, A.; Meade, M.O.; Parry, N.G.; Forbes, T.L. The effect of loss to follow-up on treatment of blunt traumatic thoracic aortic injury. J. Vasc. Surg. 2015, 61, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Dettori, J.R. Loss to follow-up. Evid. Based Spine Care J. 2011, 2, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Temeck, B.K.; Kranda, K.; Thomas, G.; Russo, A.; Smith, P.; Friauf, W.; Steinberg, S.M. Phase, III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann. Surg. Oncol. 1997, 4, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S. Photodynamic therapy for malignant pleural mesothelioma. J. Natl. Compr. Cancer Netw. 2012, 10 (Suppl. 2), S75–S79. [Google Scholar]
- Friedberg, J.S. Photodynamic therapy for malignant pleural mesothelioma: The future of treatment? Expert Rev. Respir. Med. 2011, 5, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Munck, C.; Mordon, S.R.; Scherpereel, A.; Porte, H.; Dhalluin, X.; Betrouni, N. Intrapleural photodynamic therapy for mesothelioma: What place and which future? Ann. Thorac. Surg. 2015, 99, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Du, K.L.; Both, S.; Friedberg, J.S.; Rengan, R.; Hahn, S.M.; Cengel, K.A. Extrapleural pneumonectomy, photodynamic therapy and intensity modulated radiation therapy for the treatment of malignant pleural mesothelioma. Cancer Biol. Ther. 2010, 10, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Opitz, I.; Krueger, T.; Pan, Y.; Altermatt, H.J.; Wagnières, G.; Ris, H.B. Preclinical comparison of mTHPC and verteporfin for intracavitary photodynamic therapy of malignant pleural mesothelioma. Eur. Surg. Res. 2006, 38, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.; Altermatt, H.J.; Mettler, D.; Scholl, B.; Magnusson, L.; Ris, H.B. Experimental photodynamic therapy for malignant pleural mesothelioma with pegylated mTHPC. Lasers Surg. Med. 2003, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Mick, R.; Stevenson, J.; Metz, J.; Zhu, T.; Buyske, J.; Sterman, D.H.; Pass, H.I.; Glatstein, E.; Hahn, S.M. A phase, I study of, Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann. Thorac. Surg. 2003, 75, 952–959. [Google Scholar] [CrossRef]
- Moskal, T.L.; Dougherty, T.J.; Urschel, J.D.; Antkowiak, J.G.; Regal, A.M.; Driscoll, D.L.; Takita, H. Operation and photodynamic therapy for pleural mesothelioma: 6-Year follow-up. Ann. Thorac. Surg. 1998, 66, 1128–1133. [Google Scholar] [CrossRef]
- Baas, P.; Murrer, L.; Zoetmulder, F.A.; Stewart, F.A.; Ris, H.B.; van Zandwijk, N.; Peterse, J.L.; Rutgers, E.J. Photodynamic therapy as adjuvant therapy in surgically treated pleural malignancies. Br. J. Cancer 1997, 76, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ris, H.B.; Giger, A.; Hof, V.I.; Mettler, D.; Stewart, J.C.; Althaus, U.; Altermatt, H.J. Experimental assessment of photodynamic therapy with chlorins for malignant mesothelioma. Eur. J. Cardiothorac. Surg. 1997, 12, 542–548. [Google Scholar] [CrossRef]
- Friedberg, J.S.; Culligan, M.J.; Mick, R.; Stevenson, J.; Hahn, S.M.; Sterman, D.; Punekar, S.; Glatstein, E.; Cengel, K. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann. Thorac. Surg. 2012, 93, 1658–1665, discussion 1665–1657. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Norberto, J.J. Multimodality management of malignant pleural mesothelioma. Chest 1998, 113, 61S–65S. [Google Scholar] [CrossRef] [PubMed]
- Kotova, S.; Wong, R.M.; Cameron, R.B. New and emerging therapeutic options for malignant pleural mesothelioma: Review of early clinical trials. Cancer Manag. Res. 2015, 7, 51–63. [Google Scholar] [PubMed]
- Schouwink, H.; Rutgers, E.T.; van der Sijp, J.; Oppelaar, H.; van Zandwijk, N.; van Veen, R.; Burgers, S.; Stewart, F.A.; Zoetmulder, F.; Baas, P. Intraoperative photodynamic therapy after pleuropneumonectomy in patients with malignant pleural mesothelioma: Dose finding and toxicity results. Chest 2001, 120, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Luketich, J.D.; Westkaemper, J.; Sommers, K.E.; Ferson, P.F.; Keenan, R.J.; Landreneau, R.J. Bronchoesophagopleural fistula after photodynamic therapy for malignant mesothelioma. Ann. Thorac. Surg. 1996, 62, 283–284. [Google Scholar] [CrossRef]
- Werner-Wasik, M.; Scott, C.; Cox, J.D.; Sause, W.T.; Byhardt, R.W.; Asbell, S.; Russell, A.; Komaki, R.; Lee, J.S. Recursive partitioning analysis of 1999 Radiation Therapy Oncology Group (RTOG) patients with locally-advanced non-small-cell lung cancer (LA-NSCLC): Identification of five groups with different survival. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1475–1482. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The, IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Bains, M.S.; Beattie, E.J., Jr. Indications for pleurectomy in malignant effusion. Cancer 1975, 35, 734–738. [Google Scholar] [CrossRef]
- Mott, F.E.; Sharma, N.; Ashley, P. Malignant pleural effusion in non-small cell lung cancer—Time for a stage revision? Chest 2001, 119, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Parvez, Z.; Regal, A.M.; Takita, H. Neoadjuvant chemotherapy and operations in the treatment of lung cancer with pleural effusion. J. Thorac. Cardiovasc. Surg. 1991, 101, 946–947. [Google Scholar] [PubMed]
- Chen, K.C.; Hsieh, Y.S.; Tseng, Y.F.; Shieh, M.J.; Chen, J.S.; Lai, H.S.; Lee, J.M. Pleural photodynamic therapy and surgery in lung cancer and thymoma patients with pleural spread. PLoS ONE 2015, 10, e0133230. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Mick, R.; Stevenson, J.P.; Zhu, T.; Busch, T.M.; Shin, D.; Smith, D.; Culligan, M.; Dimofte, A.; Glatstein, E. Phase, II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J. Clin. Oncol. 2004, 22, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidane, B.; Hirpara, D.; Yasufuku, K. Photodynamic Therapy in Non-Gastrointestinal Thoracic Malignancies. Int. J. Mol. Sci. 2016, 17, 135. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010135
Kidane B, Hirpara D, Yasufuku K. Photodynamic Therapy in Non-Gastrointestinal Thoracic Malignancies. International Journal of Molecular Sciences. 2016; 17(1):135. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010135
Chicago/Turabian StyleKidane, Biniam, Dhruvin Hirpara, and Kazuhiro Yasufuku. 2016. "Photodynamic Therapy in Non-Gastrointestinal Thoracic Malignancies" International Journal of Molecular Sciences 17, no. 1: 135. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010135