Survivin Modulates Squamous Cell Carcinoma-Derived Stem-Like Cell Proliferation, Viability and Tumor Formation in Vivo
Abstract
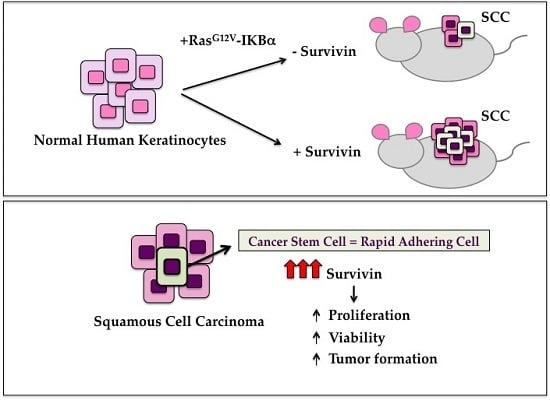
:1. Introduction
2. Results and Discussion
2.1. Survivin Expression Affects cSCC Severity in Vitro

2.2. Survivin Downregulation Decreases SCC Aggressiveness in 3D-Models

2.3. Survivin Expression Is Crucial in Tumor Aggressiveness

| Condition | Tumour at 4 Weeks | Tumour at 6 Weeks | Tumour Size (cm3 ± SD) |
|---|---|---|---|
| NoLacZ Scramble | − (3/3) | + (3/3) | 0.49 ± 0.17 |
| NoLacZ Survivin siRNA | − (3/3) | + (3/3) | 0.39 ± 0.14 |
| RasG12V-IκBαM Scramble | + (3/3) | + (3/3) | 0.89 ± 0.33 |
| RasG12V-IκBαM Survivin siRNA | − (3/3) | + (3/3) | 1.01 ± 0.38 |

3. Materials and Methods
3.1. Isolation of Primary Keratinocytes from cSCC Tissues
3.2. Isolation of Primary Keratinocytes from Healthy Skin and SCC Cell Line Culture
3.3. siRNA Transfection of Keratinocytes
3.4. Detection of Cell Viability by MTT
3.5. Colony Forming Efficiency (CFE)
3.6. Western Blotting (WB)
3.7. H and E Staining and Mitotic Index Calculation
3.8. Immunohistochemistry
3.9. Immunofluorescence
3.10. Skin Reconstruct
3.11. In Vivo Tumorigenesis
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mydlarz, W.K.; Weber, R.S.; Kupferman, M.E. Cutaneous malignancy of the head and neck. Surg. Oncol. Clin. N. Am. 2015, 24, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Ma, C.; Nie, X.; Lu, J.; Lenarz, M.; Kaufmann, A.M.; Albers, A.E. Biology and immunology of cancer stem(-like) cells in head and neck cancer. Crit. Rev. Oncol. Hematol. 2015, 95, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, B.B. Tumor-initiating and -propagating cells: Cells that we would like to identify and control. Neoplasia 2010, 12, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.E.; Chen, C.; Köberle, B.; Qian, X.; Klussmann, J.P.; Wollenberg, B.; Kaufmann, A.M. Stem cells in squamous head and neck cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Meeran, S.M. Epigenetics of cancer stem cells: Pathways and therapeutics. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J. Keratinocyte stem cells: Targets for cutaneous carcinogens. J. Clin. Investig. 2000, 106, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lichti, U.; Anders, J.; Yuspa, S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 2008, 3, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Dajee, M.; Tarutani, M.; Deng, H.; Cai, T.; Khavari, P.A. Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene 2002, 21, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Dajee, M.; Lazarov, M.; Zhang, J.Y.; Cai, T.; Green, C.L.; Russell, A.J.; Marinkovich, M.P.; Tao, S.; Lin, Q.; Kubo, Y.; et al. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 2003, 421, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Lapouge, G.; Youssef, K.K.; Vokaer, B.; Achouri, Y.; Michaux, C.; Sotiropoulou, P.A.; Blanpain, C. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 7431–7436. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.W.; Jensen, K.B.; Trotter, M.W.; Connelly, J.T.; Broad, S.; Watt, F.M. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development 2013, 140, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplyfying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef]
- Tiberio, R.; Marconi, A.; Fila, C.; Fumelli, C.; Pignatti, M.; Krajewski, S.; Giannetti, A.; Reed, J.C.; Pincelli, C. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002, 524, 139–144. [Google Scholar] [CrossRef]
- Li, A.; Kaur, P. FACS enrichment of human keratinocyte stem cells. Methods Mol. Biol. 2005, 289, 87–96. [Google Scholar] [PubMed]
- Marconi, A.; Dallaglio, K.; Lotti, R.; Vaschieri, C.; Truzzi, F.; Fantini, F.; Pincelli, C. Survivin identifies keratinocyte stem cells and is downregulated by anti-β1 integrin during anoikis. Stem Cells 2007, 25, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Colnaghi, R.; Connell, C.M.; Barrett, R.M.; Wheatley, S.P. Separating the anti-apoptotic and mitotic roles of survivin. J. Biol. Chem. 2006, 281, 33450–33456. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Beltrami, E.; Wall, N.R.; Plescia, J.; Altieri, D.C. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Investig. 2004, 114, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Gierth, M.; Shalekenov, S.; Aziz, A.; Schäfer, J.; Burger, M.; Denzinger, S.; Hofstädter, F.; Giedl, C.; Otto, W. Epithelial-mesenchymal transformation markers E-cadherin and survivin predict progression of stage pTa urothelial bladder carcinoma. Available online: http://0-link-springer-com.brum.beds.ac.uk/article/10.1007/s00345-015-1690-5 (accessed on 22 September 2015).
- Xie, S.; Xu, H.; Shan, X.; Liu, B.; Wang, K.; Cai, Z. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: Evidence from a meta-analysis. PLoS ONE 2015, 10, e0116517. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, J.; Feng, Z.; Fan, W.; Wang, Y.; Li, J.; Tong, D. The expression of Survivin and NF-κB associated with prognostically worse clinicopathologic variables in hepatocellular carcinoma. Tumour Biol. 2014, 35, 9905–9910. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, U.; Zaidi, A.H.; Kosovec, J.E.; Kasi, P.M.; Komatsu, Y.; Rotoloni, C.L.; Davison, J.M.; Irvin, R.C.; Hoppo, T.; Nason, K.S.; et al. Prognostic value and targeted inhibition of survivin expression in esophageal adenocarcinoma and cancer-adjacent squamous epithelium. PLoS ONE 2013, 8, e78343. [Google Scholar] [CrossRef] [PubMed]
- Dallaglio, K.; Petrachi, T.; Marconi, A.; Truzzi, F.; Lotti, R.; Saltari, A.; Morandi, P.; Puviani, M.; Maiorana, A.; Pincelli, C. Expression of nuclear survivin in normal skin and squamous cell carcinoma: A possible role in tumour invasion. Br. J. Cancer 2014, 110, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.; Zhou, S.; Liu, Y.; Song, D.; Luan, Z.; Dai, X.; Li, Y.; Tang, N.; Wen, J.; Li, L. Sox2 protects neural stem cells from apoptosis via up-regulating survivin expression. Biochem. J. 2013, 450, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Qiu, Y.; Huang, X.; Diao, L.; Zhang, N.; Coombes, K.R.; Mak, D.H.; Konopleva, M.; Cortes, J.; Kantarjian, H.M.; et al. Survivin is highly expressed in CD34+38− leukemic stem/progenitor cells and predicts poor clinical outcomes in AML. Blood 2012, 120, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dallaglio, K.; Petrachi, T.; Marconi, A.; Truzzi, F.; Lotti, R.; Saltari, A.; Morandi, P.; Puviani, M.; Maiorana, A.; Roop, D.R.; et al. Isolation and characterization of squamous cell carcinoma-derived stem-like cells: Role in tumor formation. Int. J. Mol. Sci. 2013, 14, 19540–19555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, S.; Rodel, C.; Mirsch, J.; Harter, P.N.; Tomicic, M.T.; Mittelbronn, M.; Kaina, B.; Rödel, F. Survivin inhibition and DNA double-strand break repair: A molecular mechanism to overcome radioresistance in glioblastoma. Radiother. Oncol. 2011, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Acquati, S.; Greco, A.; Licastro, D.; Bhagat, H.; Ceric, D.; Rossini, Z.; Grieve, J.; Shaked-Rabi, M.; Henriquez, N.V.; Brandner, S.; et al. Epigenetic regulation of survivin by Bmi1 is cell type specific during corticogenesis and in gliomas. Stem Cells 2013, 31, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Reers, S.; Pfannerstill, A.C.; Maushagen, R.; Pries, R.; Wollenberg, B. Stem cell profiling in head and neck cancer reveals an Oct-4 expressing subpopulation with properties of chemoresistance. Oral Oncol. 2014, 50, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Klonisch, T.; Wiechec, E.; Hombach-Klonisch, S.; Ande, S.R.; Wesselborg, S.; Schulze-Osthoff, K.; Los, M. Cancer stem cell markers in common cancers-therapeutic implications. Trends Mol. Med. 2008, 14, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Morandi, P.; Lotti, R.; Saltari, A.; Truzzi, F.; Schnebert, S.; Dumas, M.; Marconi, A.; Pincelli, C. Notch cooperates with survivin to maintain stemness and to stimulate proliferation in human keratinocytes during ageing. Int. J. Mol. Sci. 2015, 16, 26291–26302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Kong, W.; Falk, A.; Hu, J.; Zhou, L.; Pollard, S.; Smith, A. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS ONE 2009, 4, e5498. [Google Scholar] [CrossRef] [PubMed]
- Commandeur, S.; de Gruijl, F.R.; Willemze, R.; Tensen, C.P.; El Ghalbzouri, A. An in vitro three-dimensional model of primary human cutaneous squamous cell carcinoma. Exp. Dermatol. 2009, 18, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wei, S.; Mitchelson, K.; Gao, Y.; Zheng, Y.; Meng, Z.; Gan, Y.; Yu, G. miR-34a inhibits migration and invasion of tongue squamous cell carcinoma via targeting MMP9 and MMP14. PLoS ONE 2014, 9, e108435. [Google Scholar] [CrossRef] [PubMed]
- Moubayed, N.; Weichenthal, M.; Harder, J.; Wandel, E.; Sticherling, M.; Glaser, R. Psoriasin (S100A7) is significantly up-regulated in human epithelial skin tumors. J. Cancer Res. Clin. Oncol. 2007, 133, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Q.; Liu, G.; Song, X.; Zhang, J. Psoriasin (S100A7) is a novel biomarker for lung squamous cell carcinoma in humans. Cancer Cell Int. 2015, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Khavari, P.A. Modelling cancer in human skin tissue. Nat. Rev. Cancer 2006, 6, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 2014, 344, 1242281. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Watt, F.M. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb. Perspect. Med. 2014, 4, a013631. [Google Scholar] [CrossRef] [PubMed]
- Pierceall, W.E.; Goldberg, L.H.; Tainsky, M.A.; Mukhopadhyay, T.; Ananthaswamy, H.N. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol. Carcinog. 1991, 4, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, M.; Kubo, Y.; Cai, T.; Dajee, M.; Tarutani, M.; Lin, Q.; Fang, M.; Tao, S.; Green, C.L.; Khavari, P.A. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat. Med. 2002, 8, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. New wirings in the survivin networks. Oncogene 2008, 27, 6276–6284. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.W.; Schamberger, C.J.; Schmidt, G.E.; Sasgary, S.; Cerni, C. Inhibitor of apoptosis protein (IAP) survivin is upregulated by oncogenic c-H-Ras. Oncogene 2003, 22, 4266–4280. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.W.; Rodgarkia-Dara, C.J.; Schreiner, C.; Holzmann, K.; Krupitza, G.; Cerni, C. Oncogenic c-H-ras deregulates survivin expression: An improvement for survival. FEBS Lett. 2007, 581, 4921–4296. [Google Scholar] [CrossRef] [PubMed]
- Tecleab, A.; Sebti, S.M. Depletion of K-Ras promotes proteasome degradation of survivin. Cell Cycle 2013, 12, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wiedemuth, R.; Klink, B.; Töpfer, K.; Schröck, E.; Schackert, G.; Tatsuka, M.; Temme, A. Survivin safeguards chromosome numbers and protects from aneuploidy independently from p53. Mol. Cancer 2014, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, H.R.; Ali, N.; Nissen, L.J.; Harfouche, G.; de Verneuil, H.; Taïeb, A.; Mazurier, F. HIF-1α in epidermis: Oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J. Investig. Dermatol. 2011, 131, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Bauer, J.; Magnusson, N.E.; Infanger, M.; Grimm, D. Biomarkers for anti-angiogenic therapy in cancer. Int. J. Mol. Sci. 2013, 14, 9338–9364. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.G.; Sun, Y.N.; Wang, C.; Jin de, J.; Liu, M. Role of the αv-integrin subunit in cell proliferation, apoptosis and tumor metastasis of laryngeal and hypopharyngeal squamous cell carcinomas: A clinical and in vitro investigation. Eur. Arch. Otorhinolaryngol. 2009, 266, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gandarillas, A.; Watt, F.M. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997, 11, 2869–2882. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Saltari, A.; Palazzo, E.; Lotti, R.; Petrachi, T.; Dallaglio, K.; Gemelli, C.; Grisendi, G.; Dominici, M.; Pincelli, C.; et al. CD271 mediates stem cells to early progeny transition in human epidermis. J. Investig. Dermatol. 2015, 135, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallaglio, K.; Marconi, A.; Pincelli, C. Survivin: A dual player in healthy and diseased skin. J. Investig. Dermatol. 2012, 132, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Krishnakumar, S.; Kanwar, R.K.; Cheung, C.H.; Kanwar, J.R. Clinical aspects for survivin: A crucial molecule for targeting drug-resistant cancers. Drug Discov. Today 2015, 20, 578–587. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotti, R.; Palazzo, E.; Petrachi, T.; Dallaglio, K.; Saltari, A.; Truzzi, F.; Quadri, M.; Puviani, M.; Maiorana, A.; Marconi, A.; et al. Survivin Modulates Squamous Cell Carcinoma-Derived Stem-Like Cell Proliferation, Viability and Tumor Formation in Vivo. Int. J. Mol. Sci. 2016, 17, 89. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010089
Lotti R, Palazzo E, Petrachi T, Dallaglio K, Saltari A, Truzzi F, Quadri M, Puviani M, Maiorana A, Marconi A, et al. Survivin Modulates Squamous Cell Carcinoma-Derived Stem-Like Cell Proliferation, Viability and Tumor Formation in Vivo. International Journal of Molecular Sciences. 2016; 17(1):89. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010089
Chicago/Turabian StyleLotti, Roberta, Elisabetta Palazzo, Tiziana Petrachi, Katiuscia Dallaglio, Annalisa Saltari, Francesca Truzzi, Marika Quadri, Mario Puviani, Antonino Maiorana, Alessandra Marconi, and et al. 2016. "Survivin Modulates Squamous Cell Carcinoma-Derived Stem-Like Cell Proliferation, Viability and Tumor Formation in Vivo" International Journal of Molecular Sciences 17, no. 1: 89. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010089