Investigation of Self-Assembly Processes for Chitosan-Based Coagulant-Flocculant Systems: A Mini-Review
Abstract
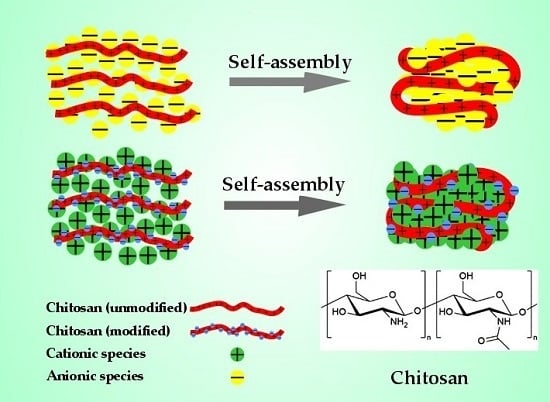
:1. Introduction
2. Use of Chitosan in Coagulation-Flocculation
2.1. Chitosan as a Coagulant
2.2. Chitosan as a Coagulant Aid
2.3. Modified Chitosan as a Coagulant
3. Application of Chitosan in Coagulation-Flocculation for Water Treatment: An Overview
3.1. Clay Particle Removal
3.2. Removal of Turbidity from Sea and Surface Water
3.3. Anionic and Cationic Dye Removal
3.4. Industrial Wastewater Treatment
3.5. Organic Cells and Biomaterial Substance Removal
3.6. Arsenic Removal
4. Effect of Operating Parameters of the Coagulation-Flocculation Process
4.1. pH Effects
4.2. Dosage Effects
4.3. Mechanical Effects
4.4. Temperature Effects
5. Effect of Chitosan Morphology
5.1. Molecular Weight
5.2. Effects of the Degree of Deacetylation
6. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, S.; Zhong, L.; Pan, J.; Ma, Q. Optimizing coagulation and flocculation process for kaolinite suspension with chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2013, 428, 100–110. [Google Scholar] [CrossRef]
- Li, J.; Song, X.; Pan, J.; Zhong, L.; Jiao, S.; Ma, Q. Adsorption and flocculation of bentonite by chitosan with varying degree of deacetylation and molecular weight. Int. J. Biol. Macromol. 2013, 62, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Lo, S.L.; Chang, C.L.; Chen, F.L.; Wu, Y.D.; Ma, J.I. Treatment of highly turbid water using chitosan and aluminum salts. Sep. Purif. Technol. 2013, 104, 322–326. [Google Scholar] [CrossRef]
- Bina, B.; Ebrahimi, A.; Hesami, F. The effectiveness of chitosan as coagulant aid in turbidity removal from water. Int. J. Environ. Health Eng. 2014. [Google Scholar] [CrossRef]
- Rojas-Reyna, R.; Schwarz, S.; Heinrich, G.; Petzold, G.; Schütze, S.; Bohrisch, J. Flocculation efficiency of modified water soluble chitosan versus commonly used commercial polyelectrolytes. Carbohydr. Polym. 2010, 81, 317–322. [Google Scholar] [CrossRef]
- Rios-Donato, N.; Navarro, R.; Avila-Rodriguez, M.; Mendizábal, E. Coagulation–flocculation of colloidal suspensions of kaolinite, bentonite, and alumina by chitosan sulfate. J. Appl. Polym. Sci. 2012, 123, 2003–2010. [Google Scholar] [CrossRef]
- Wang, J.P.; Chen, Y.Z.; Yuan, S.J.; Sheng, G.P.; Yu, H.Q. Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res. 2009, 43, 5267–5275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, Y.; Huang, X.; Chen, Y.; Lu, Y.; Chen, A.; Jiang, Y.; Gu, W.; Qian, X.; Yang, H.; et al. Cationic content effects of biodegradable amphoteric chitosan-based flocculants on the flocculation properties. J. Environ. Sci. 2012, 24, 1378–1385. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yan, H.; Wu, H.; Yang, H.; Wu, Q.; Li, H.; Li, A.; Cheng, R. Evaluation of a novel chitosan-based flocculant with high flocculation performance, low toxicity and good floc properties. J. Hazard. Mater. 2014, 276, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, Y.; Lu, Y.; Chen, Y.; Huang, X.; Chen, A.; Jiang, Y.; Gu, W.; Qian, X.; Yang, H.; et al. Flocculation properties of biodegradable amphoteric chitosan-based flocculants. Chem. Eng. J. 2011, 172, 287–295. [Google Scholar] [CrossRef]
- Bergamasco, R.; Konradt-Moraes, L.C.; Vieira, M.F.; Fagundes-Klen, M.R.; Vieira, A.M.S. Performance of a coagulation-ultrafiltration hybrid process for water supply treatment. Chem. Eng. J. 2011, 166, 483–489. [Google Scholar] [CrossRef]
- Zemmouri, H.; Drouiche, M.; Sayeh, A.; Lounici, H.; Mameri, N. Coagulation flocculation test of keddara’s water dam using chitosan and sulfate aluminium. Procedia Eng. 2012, 33, 254–260. [Google Scholar] [CrossRef]
- Altaher, H. The use of chitosan as a coagulant in the pre-treatment of turbid sea water. J. Hazard. Mater. 2012, 233, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Meraz, K.A.S.; Vargas, S.M.P.; Maldonado, J.T.L.; Bravo, J.M.C.; Guzman, M.T.O.; Maldonado, E.A.L. Eco-friendly innovation for nejayote coagulation-flocculation process using chitosan: Evaluation through Zeta (ζ) potential measurements. Chem. Eng. J. 2016, 284, 536–542. [Google Scholar] [CrossRef]
- Leiviskä, T.; Sarpola, A.; Tanskanen, J. Removal of lipophilic extractives from debarking wastewater by adsorption on kaolin or enhanced coagulation with chitosan and kaolin. Appl. Clay Sci. 2012, 61, 22–28. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Belgiorno, V. Olive mill and winery wastewaters pre-treatment by coagulation with chitosan. Sep. Sci. Technol. 2010, 45, 2447–2452. [Google Scholar] [CrossRef]
- Saeed, A.; Fatehi, P.; Ni, Y. Chitosan as a flocculant for pre-hydrolysis liquor of kraft-based dissolving pulp production process. Carbohydr. Polym. 2011, 86, 1630–1636. [Google Scholar] [CrossRef]
- Zemmouri, H.; Kadouche, S.; Lounici, H.; Hadioui, M.; Mameri, N. Use of chitosan in coagulation flocculation of raw water of Keddara and Beni Amrane dams. Water Sci. Technol. 2011, 11, 202–210. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Grassi, M.; Belgiorno, V. Pre-treatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep. Purif. Technol. 2008, 63, 648–653. [Google Scholar] [CrossRef]
- Akdemir, E.O. A statistical experiment design approach for decolorization of textile dyestuff by coagulation with chitosan. Fresenius Environ. Bull. 2012, 21, 6. [Google Scholar]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 254, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Szyguła, A.; Guibal, E.; Palacín, M.A.; Ruiz, M.; Sastre, A.M. Removal of an anionic dye (Acid Blue 92) by coagulation–flocculation using chitosan. J. Environ. Manag. 2009, 90, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Bina, B.; Mehdinejad, M.H.; Nikaaeen, M.; Attar, H.M. Effectiveness of chitosan as natural coagulant aid in treating turbid water. Iran. J. Environ. Health. Sci. Eng. 2009, 6, 247–252. [Google Scholar]
- Bina, B.; Ebrahimi, A.; Hesami, F.; Amin, M. Arsenic removal by coagulation using ferric chloride and chitosan from water. Int. J. Environ. Health Eng. 2013. [Google Scholar] [CrossRef]
- Del Carmen Cuéllar Martínez, T.; Rodríguez, R.A.; Voltolina, D.; Morquecho, L. Effectiveness of coagulants-flocculants for removing cells and toxins of Gymnodinium catenatum. Aquaculture 2016, 452, 188–193. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Wang, P.; Qian, J.; Hou, J.; Ao, Y.; Wu, B. The performance of chitosan/montmorillonite nanocomposite during the flocculation and floc storage processes of Microcystis aeruginosa cells. Environ. Sci. Pollut. Res. 2015, 22, 11148–11161. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Y.; Huang, X.; Chen, A.; Yang, Z.; Jiang, Y.; Cai, J.; Gu, W.; Qian, X.; Yang, H.; et al. Preparation of strong cationic chitosan-graft-polyacrylamide flocculants and their flocculating properties. Ind. Eng. Chem. Res. 2011, 50, 7141–7149. [Google Scholar] [CrossRef]
- Lagaly, G. From Clay Mineral Crystals to Colloidal Clay Mineral Dispersions. In Coagulation and Flocculation: Theory and Applications; Dobiás, B., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 427–430. [Google Scholar]
- Segad, M.; Jönsson, B.; Åkesson, T.; Cabane, B. Ca/Na Montmorillonite: Structure, forces and swelling properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Baskan, M.B.; Pala, A. Determination of arsenic removal efficiency by ferric ions using response surface methodology. J. Hazard. Mater. 2009, 166, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. Sources and behaviour of arsenic in natural water. In United Nations Synthesis Report on Arsenic in Drinking Water; 2005; pp. 1–61. Available online: www.who.int/water_sanitation_health/dwq/arsenicun1.pdf (accessed on 28 September 2016).
- Bilici Baskan, M.; Pala, A. A statistical experiment design approach for arsenic removal by coagulation process using aluminum sulfate. Desalination 2010, 254, 42–48. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Q.; Liu, H.; Qu, J. Coagulation of methylated arsenic from drinking water: Influence of methyl substitution. J. Hazard. Mater. 2015, 293, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.; Katsoyiannis, I. Removal of arsenates from contaminated water by coagulation-direct filtration. Sep. Sci. Technol. 2002, 37, 2859–2873. [Google Scholar] [CrossRef]
- Pallier, V.; Feuillade-Cathalifaud, G.; Serpaud, B.; Bollinger, J.C. Effect of organic matter on arsenic removal during coagulation/flocculation treatment. J. Colloid Interface Sci. 2010, 342, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.I.; Stebbins, D.M.; Alcantar, N.A. Combining ferric salt and cactus mucilage for arsenic removal from water. Environ. Sci. Technol. 2016, 50, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, H.; Chen, G.; Qu, J. Effect of aluminum speciation on arsenic removal during coagulation process. Sep. Purif. Technol. 2012, 86, 35–40. [Google Scholar] [CrossRef]
- Zhang, W.; Shang, Y.; Yuan, B.; Jiang, Y.; Lu, Y.; Qin, Z.; Chen, A.; Qian, X.; Yang, H.; Cheng, R. The flocculating properties of chitosan-graft-polyacrylamide flocculants (II)—Test in pilot scale. J. Appl. Polym. Sci. 2010, 117, 2016–2024. [Google Scholar] [CrossRef]
- Akdemir, E.O.; Ozer, A. Statistical optimization of process parameters for ultrafiltration of olive oil mill wastewaters. Desalin. Water Treat. 2013, 51, 5987–5995. [Google Scholar] [CrossRef]
- Wilson, L.D. An overview of coagulation-flocculation technology. Water Cond. Purific Mag. 2014, 56, 28–34. [Google Scholar]
- Zhang, X.; Gu, W.J.; Li, H.; Chi, H.; Chen, L. Flocculation of reed pulp suspensions by quaternary chitosan-nanoparticle SiO2 retention aid systems. J. Appl. Polym. Sci. 2010, 117, 742–749. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Kluwer Academic Publishers: Dordrecht, The Netherlands; Amsterdam, The Netherlands, 1999. [Google Scholar]
- Bárány, S.; Meszaros, R.; Marcinova, L.; Skvarla, J. Effect of polyelectrolyte mixtures on the electrokinetic potential and kinetics of flocculation of clay mineral particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 48–55. [Google Scholar] [CrossRef]
- Boddohi, S.; Moore, N.; Johnson, P.A.; Kipper, M.J. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 2009, 10, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, C.L.; Bezerril, P.M.; dos Santos, D.E.S.; Dantas, T.N.C.; Pereira, M.R.; Fonseca, J.L.C. Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly(methacrylic acid) and chitosan. Biomacromolecules 2006, 7, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Rahmanifar, B.; Moradi Dehaghi, S. Removal of organochlorine pesticides by chitosan loaded with silver oxide nanoparticles from water. Clean Technol. Environ. Policy 2013, 16, 1781–1786. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption characteristics of congo red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Mohamed, M.W.L. Kinetic uptake studies of powdered materials in solution. J. Nanomater. 2015, 5, 969–980. [Google Scholar] [CrossRef]







| Coagulant | Coagulant Aid | Contaminant | Percentage Removal | Chitosan Dosage | Reference |
|---|---|---|---|---|---|
| Chitosan | – | Tortilla waste turbidity | 80% | 47 mg/g Chitosan to solid waste | [6] |
| Aluminum chloride (1017 mg/L) | Chitosan-g-PDMC | Pulp mill waste turbidity | 99.4% | 17.8 mg/L (pH 7.1) | [11] |
| Chitosan | – | Sea water turbidity | 95%–98% | 6–60 mg/L | [17] |
| Chitosan | Kaolinite (2 g/L) | Lipophilic extractives | 91% | 60 mg/L | [18] |
| Chitosan | – | Reactive Yellow 15 dye | 60%–80% | 100 mg/L | [23] |
| Chitosan | – | Furfural | 50%–55% | 0.5 mg/g Chitosan to PHL | [20] |
| CMC-g-PAM11 | – | Basic Bright Yellow 7GL dye | 95% | 160 mg/L (pH 11) | [24] |
| Alum (5 mg/L) | Chitosan | Iranian surface water turbidity | 98% | 0.5 mg/L | [26] |
| Iron(III) chloride (30 mg/L) | Chitosan | Arsenate species | 100% | 0.5 mg/L (pH 7) | [27] |
| Chitosan | CaO (0.3 g/L) | Extracellular toxins | Ineffective | 0.075 g/L | [28] |
| CMC-g-PAM11 | – | Kaolin | 95% | 16 mg/L (pH 11) | [29] |
| CTS/NMMT | – | M. aeruginosa | 94.9% | 310 mg/L | [30] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalkaran, S.; Wilson, L.D. Investigation of Self-Assembly Processes for Chitosan-Based Coagulant-Flocculant Systems: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101662
Bhalkaran S, Wilson LD. Investigation of Self-Assembly Processes for Chitosan-Based Coagulant-Flocculant Systems: A Mini-Review. International Journal of Molecular Sciences. 2016; 17(10):1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101662
Chicago/Turabian StyleBhalkaran, Savi, and Lee D. Wilson. 2016. "Investigation of Self-Assembly Processes for Chitosan-Based Coagulant-Flocculant Systems: A Mini-Review" International Journal of Molecular Sciences 17, no. 10: 1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101662