Disease Activity and Conversion into Multiple Sclerosis after Optic Neuritis Is Treated with Erythropoietin
Abstract
:1. Introduction
2. Results
2.1. Baseline Status
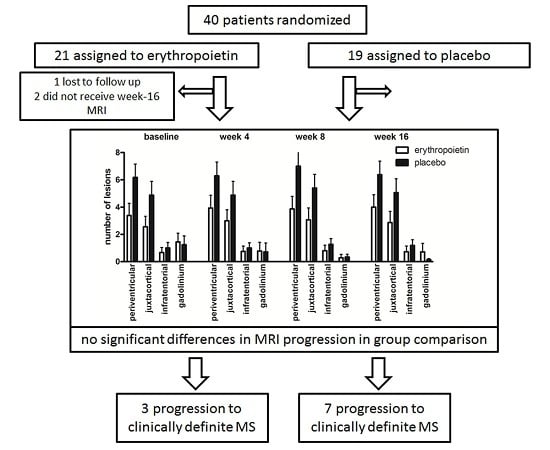
2.2. Changes in Cerebral Lesion Load
2.3. Conversion into MS
3. Discussion
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suhs, K.W.; Hein, K.; Sattler, M.B.; Gorlitz, A.; Ciupka, C.; Scholz, K.; Käsmann-Kellner, B.; Papanagiotou, P.; Schäffler, N.; Restemeyer, C.; et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann. Neurol. 2012, 72, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.P.; Altmann, D.R.; Trip, A.S.; Kallis, C.; Jones, S.J.; Schlottmann, P.G.; Garway-Heath, D.F.; Plant, G.T.; Miller, D.H. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain 2010, 133, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Filippi, M.; Stuve, O.; Waxman, S.G.; Corboy, J.; Phillips, J.T.; Lucchinetti, C.; Wilken, J.; Karandikar, N.; Hemmer, B.; et al. Characterizing the mechanisms of progression in multiple sclerosis: Evidence and new hypotheses for future directions. Arch. Neurol. 2005, 62, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Lipkin, E.; Chodkowski, B.; Reich, D.S.; Smith, S.A.; Pulicken, M.; Balcer, L.J.; Frohman, E.M.; Cutter, G.; Calabresi, P.A. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007, 69, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.B.; Merkler, D.; Maier, K.; Stadelmann, C.; Ehrenreich, H.; Bahr, M.; Diem, R. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004, 11, S181–S192. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Desai, R.A.; Bloomfield, P.S.; McIntosh, P.R.; Chapple, K.J.; Linington, C.; Fairless, R.; Diem, R.; Kasti, M.; Murphy, M.P.; et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013, 74, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, I.E.; McDonald, W.I.; du Boulay, G.H.; Kendall, B.E.; Moseley, I.F.; Halliday, A.M.; Kakigi, R.; Kriss, A.; Peringer, E. Disseminated lesions at presentation in patients with optic neuritis. J. Neurol. Neurosurg. Psychiatry 1986, 49, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Ormerod, I.E.; McDonald, W.I.; MacManus, D.G.; Kendall, B.E.; Kingsley, D.P.; Moseley, I.F. The early risk of multiple sclerosis after optic neuritis. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, J.I.; Thompson, A.J.; Kingsley, D.P.; MacManus, D.G.; Kendall, B.E.; Rudge, P.; McDonald, W.I.; Miller, D.H. The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain 1998, 121, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Rio, J.; Nos, C.; Grive, E.; Tellez, N.; Pelayo, R.; Comabella, M.; Sastre-Garriga, J.; Montalban, X. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology 2006, 67, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Battaglini, M.; Rocca, M.A.; de Leucio, A.; Absinta, M.; van Schijndel, R.; Rovira, A.; Tintoré, M.; Chard, D.; Ciccarelli, O.; et al. Location of brain lesions predicts conversion of clinically isolated syndromes to multiple sclerosis. Neurology 2013, 80, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Fisniku, L.K.; Brex, P.A.; Altmann, D.R.; Miszkiel, K.A.; Benton, C.E.; Lanyon, R.; Thompson, A.J.; Miller, D.H. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008, 131, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Frohman, T.C.; Zee, D.S.; McColl, R.; Galetta, S. The neuro-ophthalmology of multiple sclerosis. Lancet. Neurol. 2005, 4, 111–121. [Google Scholar] [CrossRef]
- Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch. Neurol. 2008, 65, 727–732. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sühs, K.-W.; Papanagiotou, P.; Hein, K.; Pul, R.; Scholz, K.; Heesen, C.; Diem, R. Disease Activity and Conversion into Multiple Sclerosis after Optic Neuritis Is Treated with Erythropoietin. Int. J. Mol. Sci. 2016, 17, 1666. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101666
Sühs K-W, Papanagiotou P, Hein K, Pul R, Scholz K, Heesen C, Diem R. Disease Activity and Conversion into Multiple Sclerosis after Optic Neuritis Is Treated with Erythropoietin. International Journal of Molecular Sciences. 2016; 17(10):1666. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101666
Chicago/Turabian StyleSühs, Kurt-Wolfram, Panagiotis Papanagiotou, Katharina Hein, Refik Pul, Kerstin Scholz, Christoph Heesen, and Ricarda Diem. 2016. "Disease Activity and Conversion into Multiple Sclerosis after Optic Neuritis Is Treated with Erythropoietin" International Journal of Molecular Sciences 17, no. 10: 1666. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101666