Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituent Resveratrol
Abstract
:1. Introduction
2. Trigeminal Pain Pathway and Spinal Trigeminal Nucleus
3. Potential Role for Resveratrol in Alleviating Nociceptive Pain
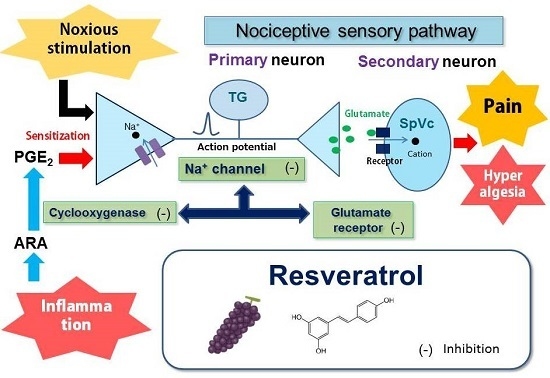
3.1. Peripheral Mechanism
3.2. Central Mechanism
4. Potential Role for Resveratrol in Alleviating Inflammatory Pain
5. Functional Significance of Pain Relief by Resveratrol and Future Directions
6. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Arranz, J.; Fraiz, N.; Sanmartin, M.; Quezada, E.; Orallo, F. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int. Immunopharmacol. 2005, 5, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Ocaña, D.Y.; Ambriz-Tututi, M.; Pérez-Severiano, F.; Granados-Soto, V. Pharmacological evidence for the participation of NO–cyclic GMP–PKG–K+ channel pathway in the antiallodynic action of resveratrol. Pharmacol. Biochem. Behav. 2006, 84, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Severiano, F.; Bermúdez-Ocaña, D.Y.; López-Sánchez, P.; Ríos, C.; Granados-Soto, V. Spinal nerve ligation reduces nitric oxide synthase activity and expression: Effect of resveratrol. Pharmacol. Biochem. Behav. 2008, 90, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.K.; Mihaliak, K.; Kroenke, K.; Bradley, J.; Tierney, W.M.; Weinberger, M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann. Intern. Med. 1999, 131, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/alternative medicine use among chronic pain clinic patients. J. PeriAnesthesia Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.I.; Genao, I.; Chen, I.; Mechaber, A.J.; Wood, J.A.; Faselis, C.J.; Kurz, J.; Menon, M.; O’Rorke, J.; Panda, M. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med. 2008, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Shir, Y.; Raja, S.N.; Weissman, C.S.; Campbell, J.N.; Seltzer, Z.E. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. J. Am. Soc. Anesthesiol. 2001, 95, 1238–1244. [Google Scholar] [CrossRef]
- Ernst, E. Complementary medicine. Curr. Opin. Rheumatol. 2003, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Tall, J.M.; Raja, S.N. Dietary constituents as novel therapies for pain. Clin. J. Pain 2004, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Im Kim, H.; Kim, T.H.; Song, J.-H. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005, 1045, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Liew, R.; Stagg, M.A.; MacLeod, K.T.; Collins, P. The red wine polyphenol, resveratrol, exerts acute direct actions on guinea-pig ventricular myocytes. Eur. J. Pharmacol. 2005, 519, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-B.; Hu, G.-Y. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res. 2005, 1056, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Granados-Soto, V.; Argüelles, C.; Ortiz, M. The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology 2002, 43, 917–923. [Google Scholar] [CrossRef]
- Gao, Z.-B.; Chen, X.-Q.; Hu, G.-Y. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006, 1111, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Hwang, S.-H.; Choi, S.-H.; Shin, T.-J.; Kang, J.; Lee, S.-M.; Nah, S.-Y. Resveratrol enhances 5-hydroxytryptamine type 3A receptor-mediated ion currents: The role of arginine 222 residue in pre-transmembrane domain I. Biol. Pharm. Bull. 2011, 34, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef] [PubMed]
- Pham-Marcou, T.A.; Beloeil, H.; Sun, X.; Gentili, M.; Yaici, D.; Benoit, G.; Benhamou, D.; Mazoit, J.-X. Antinociceptive effect of resveratrol in carrageenan-evoked hyperalgesia in rats: Prolonged effect related to COX-2 expression impairment. Pain 2008, 140, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Geisslinger, G.; Rümenapp, P.; Weiretter, F.; Szelenyi, I.; Brune, K.; Schaible, H.-G. Antinociceptive effects of R(−)- and S(+)-flurbiprofen on rat spinal dorsal horn neurons rendered hyperexcitable by an acute knee joint inflammation. J. Pharm. Exp. Ther. 1995, 275, 618–628. [Google Scholar]
- Baba, H.; Kohno, T.; Moore, K.A.; Woolf, C.J. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J. Neurosci. 2001, 21, 1750–1756. [Google Scholar] [PubMed]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Torres-López, J.E.; Ortiz, M.I.; Castaneda-Hernández, G.; Alonso-López, R.; Asomoza-Espinosa, R.; Granados-Soto, V. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 2002, 70, 1669–1676. [Google Scholar] [CrossRef]
- Gentilli, M.; Mazoit, J.X.; Bouaziz, H.; Fletcher, D.; Casper, R.F.; Benhamou, D.; Savouret, J.-F. Resveratrol decreases hyperalgesia induced by carrageenan in the rat hind paw. Life Sci. 2001, 68, 1317–1321. [Google Scholar] [CrossRef]
- Brune, K.; Zeilhofer, H. Antipyretic analgesics: Basic aspects. In Textbook of Pain; Elsevier: Amsterdam, The Netherlands, 2006; pp. 459–469. [Google Scholar]
- Sessle, B.J. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. 2000, 11, 57–91. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J. Oral Biosci. 2011, 53, 318–329. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Matsumoto, S. Change in mechanical receptive field properties induced by GABAA receptor activation in the trigeminal spinal nucleus caudalis neurons in rats. Exp. Brain Res. 2000, 134, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanimoto, T.; Ito, M.; Nasu, M.; Matsumoto, S. Role of capsaicin-sensitive primary afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp. Brain Res. 2005, 160, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J. Peripher. Nerv. Syst. 2012, 17, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Takeda, M.; Tanimoto, T.; Matsumoto, S. Convergence of nociceptive information from temporomandibular joint and tooth pulp afferents on C1 spinal neurons in the rat. Life Sci. 2004, 75, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Kogure, Y.; Yamamoto, S.; Noguchi, K.; Dai, Y. Modulation of TRP channels by resveratrol and other stilbenoids. Mol. Pain 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fang, P.; Hu, Z.; Ling, Y.; Liu, H. Mechanotransduction of trigeminal ganglion neurons innervating inner walls of rat anterior eye chambers. Am. J. Physiol.–Cell Physiol. 2015, 309, C1–C10. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Shibuya, E.; Takehana, S.; Sekiguchi, K.; Oshima, K.; Kamata, H.; Karibe, H.; Takeda, M. Local administration of resveratrol inhibits excitability of nociceptive wide-dynamic range neurons in rat trigeminal spinal nucleus caudalis. Brain Res. Bull. 2016, 124, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Takehana, S.; Sekiguchi, K.; Inoue, M.; Kubota, Y.; Ito, Y.; Yui, K.; Shimazu, Y.; Takeda, M. Systemic administration of resveratrol suppress the nociceptive neuronal activity of spinal trigeminal nucleus caudalis in rats. Brain Res. Bull. 2016, 120, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Sharma, M.; Briyal, S. Antinociceptive effect of trans-resveratrol in rats: Involvement of an opioidergic mechanism. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Chieng, B.; Christie, M. Hyperpolarization by opioids acting on μ-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br. J. Pharmacol. 1994, 113, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gebahrts, G.F.; Randich, A. Brain stem modulation of nociception. In Brain Stem Mechanisms of Behavior; Klemm, W.R., Vertes, R.P., Eds.; Wiley-Intersciences: New York, NY, USA, 1990; pp. 315–352. [Google Scholar]
- Takeda, M.; Tanimoto, T.; Nishikawa, T.; Ikeda, M.; Yoshida, S.; Ito, M.; Matsumoto, S. Volume expansion suppresses the tooth-pulp evoked jaw-opening reflex related activity of trigeminal neurons in rats. Brain Res. Bull. 2002, 58, 83–89. [Google Scholar] [CrossRef]
- Tanimoto, T.; Takeda, M.; Nishikawa, T.; Matsumoto, S. The role of 5-HT3 receptors in the vagal afferent activation-induced inhibition of C1 spinal neurons projected from tooth-pulp in the rat. J. Pharmacol. Exp. Ther. 2004. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Takeda, M.; Tanimoto, T.; Katsuumi, I.; Matsumoto, S. Tooth-pulp-evoked rostral spinal trigeminal nucleus neuron activity is inhibited by conditioning sciatic nerve stimulation in the rat: Possible role of 5-HT3 receptor mediated GABAergic inhibition. Brain Res. Bull. 2005, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Takehana, S.; Shibuya, E.; Matsuzawa, N.; Hidaka, S.; Kanai, Y.; Inoue, M.; Kubota, Y.; Shimazu, Y.; Takeda, M. Resveratrol attenuates inflammation-induced hyperexcitability of trigeminal spinal nucleus caudalis neurons associated with hyperalgesia in rats. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kadoi, J.; Takeda, M.; Matsumoto, S. Prostaglandin E2 potentiates the excitability of small diameter trigeminal root ganglion neurons projecting onto the superficial layer of the cervical dorsal horn in rats. Exp. Brain Res. 2007, 176, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Beiche, F.; Scheuerer, S.; Brune, K.; Geisslinger, G.; Goppelt-Struebe, M. Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996, 390, 165–169. [Google Scholar] [CrossRef]
- Ek, T.; Jarfelt, M.; Mellander, L.; Abrahamsson, J. Proinflammatory cytokines mediate the systemic inflammatory response associated with high-dose cytarabine treatment in children. Med. Pediatr. Oncol. 2001, 37, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Lippross, S.; Neububer, W.L.; Zeilhofer, H.U. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat. Neurosci. 2002, 5, 34–40. [Google Scholar]
- Iwata, K.; Tashiro, A.; Tsuboi, Y.; Imai, T.; Sumino, R.; Morimoto, T.; Dubner, R.; Ren, K. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J. Neurophysiol. 1999, 82, 1244–1253. [Google Scholar] [PubMed]
- Imbe, H.; Iwata, K.; Zou, S.; Dubner, R.; Ren, K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Cells Tissues Organs 2001, 169, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Curter, M.F.; Yarnitsky, D. The development of cutaneous allodynia during a migraine attack: Clinical evidence for the sequential of spinal and supraspinal nociceptive neurons in maigraine. Brain 2000, 123, 1703–1709. [Google Scholar]
- Sorkin, L.S.; Wallace, M.S. Acute pain mechanisms. Surg. Clin. N. Am. 1999, 79, 213–229. [Google Scholar] [CrossRef]
- Roch, M.; Messlinger, K.; Kulchitsky, V.; Tichonovich, O.; Azev, O.; Koulchitsky, S. Ongoing activity in trigeminal wide-dynamic range neurons is driven from the periphery. Neuroscience 2007, 150, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol. Pain 2010, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Davis, R.B.; Foster, D.F.; van Rompay, M.I.; Walters, E.E.; Wilkey, S.A.; Kaptchuk, T.J.; Eisenberg, D.M. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann. Intern. Med. 2001, 135, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rivat, C.; Richebé, P.; Laboureyras, E.; Laulin, J.-P.; Havouis, R.; Noble, F.; Moulinoux, J.-P.; Simonnet, G. Polyamine deficient diet to relieve pain hypersensitivity. PAIN® 2008, 137, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Perkins, F.M.; Kehlet, H. Chronic pain as an outcome of surgery: A review of predictive factors. J. Am. Soc. Anesthesiol. 2000, 93, 1123–1133. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Locher-Claus, M.T.; Erickson, T.E.; Law, A.S.; Johnson, W.T.; Gebhart, G. Effects of pre-emptive morphine, ibuprofen or local anesthetic on Fos expression in the spinal trigeminal nucleus following tooth pulp exposure in the rat. J. Endod. 2005, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Tillu, D.V.; Melemedjian, O.K.; Asiedu, M.N.; Qu, N.; de Felice, M.; Dussor, G.; Price, T.J. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol. Pain 2012, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Kanai, A.; Suzuki, A.; Kobayashi, M.; Hoka, S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br. J. Anaesth. 2006, 97, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Han, K.R.; Kim, C.; Chae, Y.; Kim, D.W. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int. J. Clin. Pract. 2008, 62, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Argyra, E.; Zis, P.; Vadalouca, A.; Siafaka, I. The effect of intravenous lidocaine on trigeminal neuralgia: A randomized double blind placebo controlled trial. ISRN Pain 2014, 2014. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, M.; Takehana, S.; Sekiguchi, K.; Kubota, Y.; Shimazu, Y. Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituent Resveratrol. Int. J. Mol. Sci. 2016, 17, 1702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101702
Takeda M, Takehana S, Sekiguchi K, Kubota Y, Shimazu Y. Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituent Resveratrol. International Journal of Molecular Sciences. 2016; 17(10):1702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101702
Chicago/Turabian StyleTakeda, Mamoru, Shiori Takehana, Kenta Sekiguchi, Yoshiko Kubota, and Yoshihito Shimazu. 2016. "Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituent Resveratrol" International Journal of Molecular Sciences 17, no. 10: 1702. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101702