Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes
Abstract
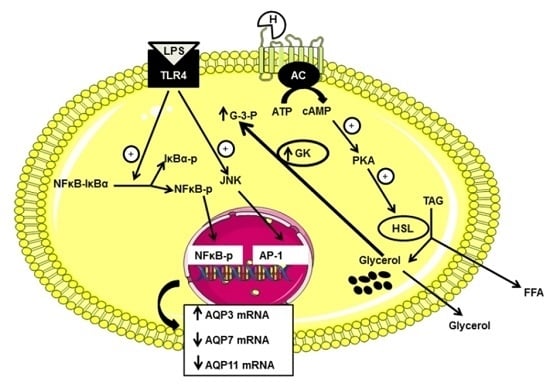
:1. Introduction
2. Results
2.1. Expression of Aquaporins (AQPs) mRNA in 3T3-L1 Cells Differentiated into Adipocytes (DC; Adipocytes) vs. Undifferentiated 3T3-L1 Cells (UDC; Preadipocytes)
2.2. Effect of Lipopolysaccharide (LPS) on AQP3, AQP7 and AQP11 mRNA Levels in DC
2.3. Effect of TLR4 Inhibition on AQP3, AQP7 and AQP11 mRNA Levels in DC
2.4. Involvement of p38, JNK and NFκB Signaling Pathways on AQP3, AQP7 and AQP11 mRNA Levels in DC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. RNA Isolation
4.4. Primer Design
4.5. Gene Expression Determined by RT-qPCR
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| CT | Control |
| DC | 3T3-L1 cells differentiated into adipocytes |
| IκBα | Inhibitors of nuclear factor κ B α |
| JNK | c-Jun N-terminal kinases |
| LPS | Lipopolysaccharide |
| NFκB | Nuclear factor-κB |
| TLR4 | Toll-like receptor 4 |
| UDC | Undifferentiated 3T3-L1 cells |
References
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2000, 104, 531–543. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Haigh, R.J.; Shepherd, P.R.; Nave, B.T.; Siddle, K. The role of phosphatidylinositol 3-kinase activity in insulin-stimulated mitogenesis in 3T3-L1 adipocytes. Biochem. Soc. Trans. 1995, 23, 179S. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett. 2006, 580, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A. Aquaporin-7 and glycerol permeability as novel obesity drug-target pathways. Trends Pharmacol. Sci. 2006, 27, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell 2005, 97, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. New members of mammalian aquaporins: AQP10-AQP12. Handb. Exp. Pharmacol. 2009, 190, 251–262. [Google Scholar] [PubMed]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Watanabe, H.; Yan, D.; Manley, G.T.; Verkman, A.S. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 2005, 118, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. 2006, 17, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 2008, 86, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Takahashi, N.; Kurata, N.; Yamaguchi, A.; Matsui, H.; Kato, S.; Takeuchi, K. Involvement of aquaporin-1 in gastric epithelial cell migration during wound repair. Biochem. Biophys. Res. Commun. 2009, 386, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through Lin7/β-catenin. PLoS ONE 2009, 4, e6167. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Rui, Y.F.; Xu, L.; Wan, C.; Li, G. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and β-catenin. Stem Cells Dev. 2014, 23, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Shimomura, I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Nagasawa, A.; Funahashi, T. Aquaporins and glycerol metabolism. Biochim. Biophys. Acta 2006, 1758, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Escote, X.; Ceperuelo-Mallafre, V.; Alcaide, M.J.; Simon, I.; Vilarrasa, N.; Wabitsch, M.; Vendrell, J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: Differences and similarities between depots. J. Clin. Endocrinol. Metab. 2010, 95, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; GarcíaNavarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux in human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Nakakoshi, M.; Hirao, A.; Imai, M.; Ishibashi, K. Mouse aquaporin 10 gene (AQP10) is a pseudogene. Biochem. Biophys. Res. Commun. 2002, 294, 630–634. [Google Scholar] [CrossRef]
- Marrades, M.P.; Milagro, F.I.; Martinez, J.A.; Moreno-Aliaga, M.J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006, 339, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; de Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Catalan, V.; Gomez-Ambrosi, J.; Pastor, C.; Rotellar, F.; Silva, C.; Rodriguez, A.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Vendrell, J.; et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008, 18, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ceperuelo-Mallafré, V.; Lecube, A.; Hernandez, C.; Chacon, M.R.; Fort, J.M.; Gallart, L.; Baena-Fustegueras, J.A.; Simó, R.; Vendrell, J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: Relation-ship with glucose abnormalities. Metabolism 2009, 58, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar]
- Ikeda, M.; Andoo, A.; Shimono, M.; Takamatsu, N.; Taki, A.; Muta, K.; Ishibashi, K. The NPC Motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J. Biol. Chem. 2011, 286, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; McMillan, R.P.; Friasrd, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant. Biochemie 2016, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int. J. Mol. Sci. 2016, 17, 1265. [Google Scholar] [CrossRef] [PubMed]
- Li, F.X.; Huang, L.Z.; Dong, C.; Wang, J.P.; Wu, H.J.; Shuang, S.M. Down-regulation of aquaporin 3 expression by lipopolysaccharide via p38/c-Jun N-terminal kinase signalling pathway in HT-29 human colon epithelial cells. World J. Gastroenterol. 2015, 21, 4547–4554. [Google Scholar] [PubMed]
- Wang, H.; Runming, J.; Tian, P.; Zhuo, Z. Enhanced expression of aquaporin-9 in rat brain edema induced by bacterial lipopolysaccharides. J. Huazhong Univ. Sci. Technol. 2009, 29, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Maeda, N.; Matsuda, M.; Ouchi, N.; Kihara, S.; Kurachi, Y.; Funahashi, T.; Matsuzawa, Y. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose specific glycerol channel. J. Biol. Chem. 2001, 276, 36251–36260. [Google Scholar]
- Maeda, N. Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome. Mol. Asp. Med. 2012, 33, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, T.; Gregoire, F.; Chiadak, J.; Courtequisse, E.; Bolaky, N.; Perret, J.; Delporte, C. Pituitary Adenylate Cyclase Activating Peptide (PACAP) Participates in adipogenesis by activating ERK signaling pathway. PLoS ONE 2013, 8, e72607. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; de Almeida, A.; de Graaf, C.; Camps, M.; Zorzano, A.; Moura, T.F.; Casini, A.; Soveral, G. A gold coordination compound as a chemical probe to unravel aquaporin-7 function. Chem. Biochem. 2014, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Mosca, A.F.; Moura, T.F.; Soveral, G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 2015, 67, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gimenez, L.; Rodriguez, A.; Balaguer, I.; Fruhbeck, G. Role of aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Hibuse, T.; Nagasawa, A.; Kishida, K.; Kuriyama, H.; Nakamura, T.; Kihara, S.; Shimomura, I.; Matsuzawa, Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 17801–17806. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.X.; Gu, X.; Pan, W.; Li, W.P.; Wei, L.; Ye, J.; Yang, L.Y.; Gu, X.J.; Ni, L.S. Over-expression of AQP7 contributes to improve insulin resistance in adipocytes. Exp. Cell Res. 2012, 318, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Banuelos, O.; Soria, A.C.; Rodriguez-Sanchez, S.; Martinez, A.; Campion, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation pattersns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Klein, J.; Lossner, U.; Klier, M.; Kralisch, S.; Paschke, R. Suppression of aquaporin adipose gene expression by isoproterenol, TNAFα, and dexamethasone. Horm. Metab. Res. 2003, 35, 222–227. [Google Scholar] [PubMed]
- Yasui, H.; Kubota, M.; Iguchi, K.; Usui, S.; Kiho, T.; Hirano, K. Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem. Biophys. Res. Commun. 2008, 373, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.E.; Larivière, N.M.; Watson, P.H.; Watson, A.J. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating Aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum. Reprod. 2009, 24, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.N.; Lee, S.H.; Lee, M.Y.; Heo, J.S.; Lee, Y.J.; Han, H.J. High glucose induced translocation of Aquaporin8 to chicken hepatocyte plasma membrane: Involvement of cAMP, PI3K/Akt, PKC, MAPKs, and microtubule. J. Cell. Biochem. 2008, 103, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Nito, C.; Kamada, H.; Endo, H.; Narasimhan, P.; Lee, Y.S.; Chan, P.H. Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. J. Neurotrauma 2012, 29, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; de Caterina, R. Relevance of new drug discovery to reduce NF-κB activation in cardiovascular disease. Vasc. Pharmacol. 2012, 57, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xia, Y.; Parker, A.S.; Verma, I.M. IKK biology. Immunol. Rev. 2012, 246, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-κB by toll like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Onnockx, S.; Vandenbroere, I.; Pirson, I. Endogenous SHIP2 does not localize in lipid rafts in 3T3-L1 adipocytes. FEBS Lett. 2004, 565, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, T.; Gregoire, F.; Delforge, V.; Delporte, C.; Perret, J. Murine 3T3L1 Adipocyte Cell Differentiation Model: Validated Reference Genes for qPCR Gene Expression Analysis. PLoS ONE 2012, 7, e37517. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Willermain, F.; Weber, C.; Bryla, A.; Salik, D.; Gregoire, F.; Bolaky, N.; Caspers, L.; Perret, J.; Delporte, C. Involvement of TonEBP/NFAT5 in osmoadaptative response of human retinal pigmented epithelial cells to hyperosmolar stress. Mol. Vis. 2016, 22, 100–115. [Google Scholar] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]




| Gene Symbol | Ct Values | p Value | |
|---|---|---|---|
| UDC | DC | ||
| AQP1 | 29.24 ± 0.81 | 24.86 ± 0.03 | 0.01 |
| AQP2 | 35.96 ± 1.86 | 35.40 ± 0.96 | 0.67 |
| AQP3 | 33.54 ± 1.11 | 31.34 ± 0.06 | 0.08 |
| AQP4 | ND | 31.04 ± 0.97 | - |
| AQP5 | ND | ND | - |
| AQP6 | ND | ND | - |
| AQP7 | ND | 24.66 ± 0.09 | - |
| AQP8 | ND | 27.66 ± 0.45 | - |
| AQP9 | 36.25 ± 0.72 | 36.24 ± 0.67 | 0.99 |
| AQP11 | 30.92 ± 0.13 | 28.82 ± 0.12 | 0.0001 |
| AQP12 | ND | ND | - |
| Genes | Primer Sequence (5′ ≥ 3′) | GenBank Accession Number |
|---|---|---|
| mmAQP1 | Forward: CCGAGACTTAGGTGGCTCAG Reverse: CCCACCCAGAAAATCCAGTG | NM_007472.2 |
| mmAQP2 | Forward: CATTGGTTTCTCTGTTACCCTG Reverse : AGAAGACCCAGTGATCATCAAAC | NM_009699.3 |
| mmAQP3 | Forward: GGGCTTCAATTCTGGCTATG Reverse: GAAGACACCAGCGATGGAAC | NM_016689.2 |
| mmAQP4 | Forward: CCAGCTCGATCTTTTGGACCCGC Reverse: GCTGCGCGGCTTTGCTGAAG | NM_009700.2 |
| mmAQP5 | Forward: TCTTGTGGGGATCTACTTCAC Reverse: AGAAGTAGAGGATTGCAGCC | NM_009701.4 |
| mmAQP6 | Forward: GGCTATGGCCTATATCGCTG Reverse: GCCAGTTGATGTGCTGTTG | NM_175087.4 |
| mmAQP7 | Forward: GCTGCTTCAGGTCCACCCACAAC Reverse: GCCACGGAACCAAGGCCAAACAC | NM_007473.4 |
| mmAQP8 | Forward: ATGGCTGGCTACTGGGACTT Reverse: CGCCAGCAGTTCTTCTTCAC | NM_007474.2 |
| mmAQP9 | Forward: TATCCCCAGAAGCCCAAACT Reverse: GCTGTTGGGATCAAACTGGA | NM_022026.3 |
| mmAQP11 | Forward: TTGCTCCTTCTGTAGGTGTG Reverse: ACTGTCCTGGGACTTAGTTC | NM_175105.3 |
| mmAQP12 | Forward: GCCCTACACATCTGCCTTC Reverse: GAGGACAGCCAGGATCATC | NM_177587.2 |
| mmNONO | Forward: TGCTCCTGTGCCACCTGGTACTC Reverse : CCGGAGCTGGACGGTTGAATGC | NM_023144.2 |
| mmACTB | Forward: CCTGTGCTGCTCACCGAGGC Reverse : GACCCCGTCTCCGGAGTCCATC | NM_007393.3 |
| mmYWHAZ | Forward: AAAAACAGCTTTCGATGAAGCC Reverse : GCCGGTTAATTTTCCCCTCC | NM_011740.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiadak, J.D.; Arsenijevic, T.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Delporte, C. Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes. Int. J. Mol. Sci. 2016, 17, 1742. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101742
Chiadak JD, Arsenijevic T, Gregoire F, Bolaky N, Delforge V, Perret J, Delporte C. Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes. International Journal of Molecular Sciences. 2016; 17(10):1742. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101742
Chicago/Turabian StyleChiadak, Jeanne Durendale, Tatjana Arsenijevic, Francoise Gregoire, Nargis Bolaky, Valerie Delforge, Jason Perret, and Christine Delporte. 2016. "Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes" International Journal of Molecular Sciences 17, no. 10: 1742. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101742