Leaf Volatile Compounds and Associated Gene Expression during Short-Term Nitrogen Deficient Treatments in Cucumis Seedlings
Abstract
:1. Introduction
2. Results and Discussion
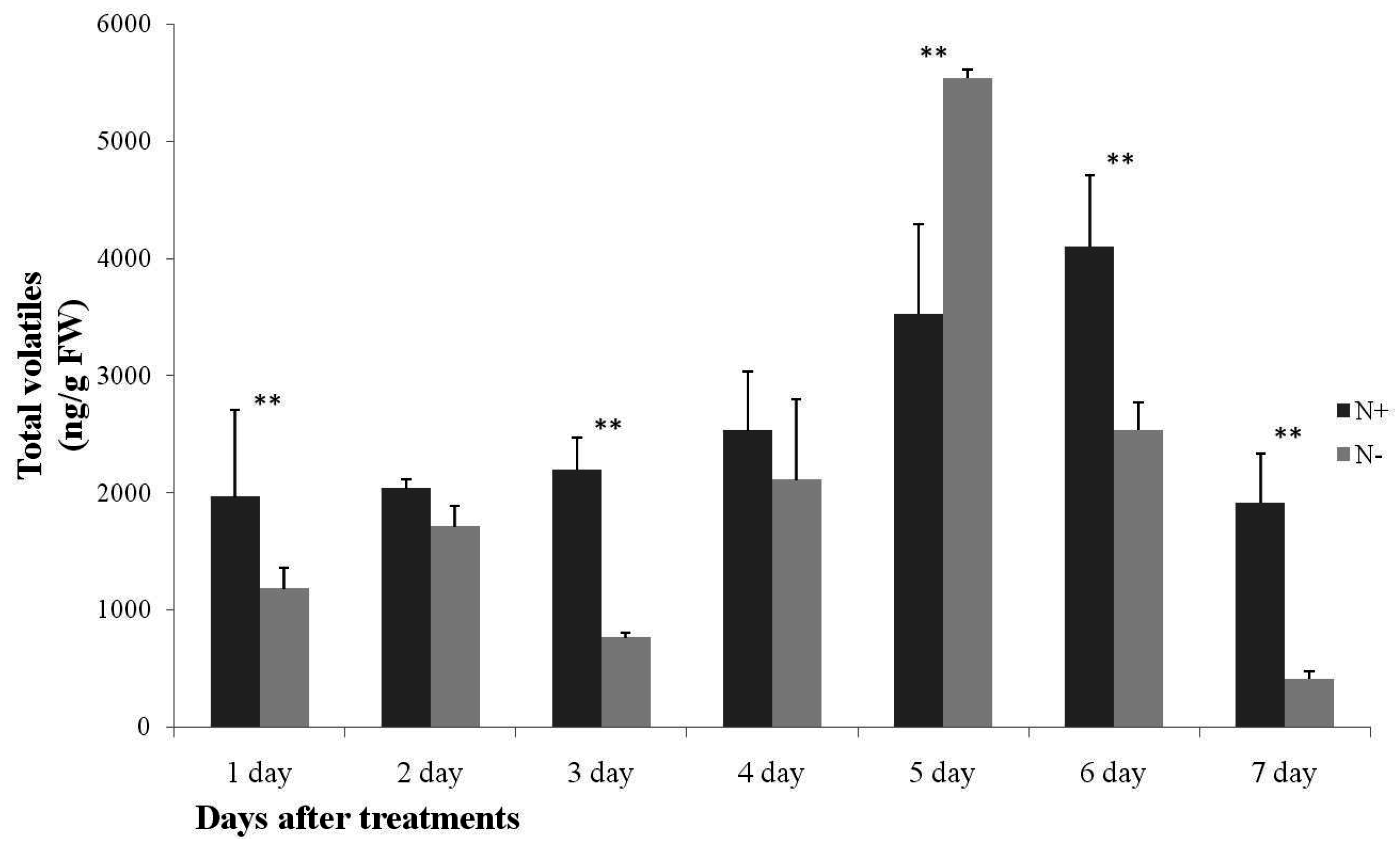
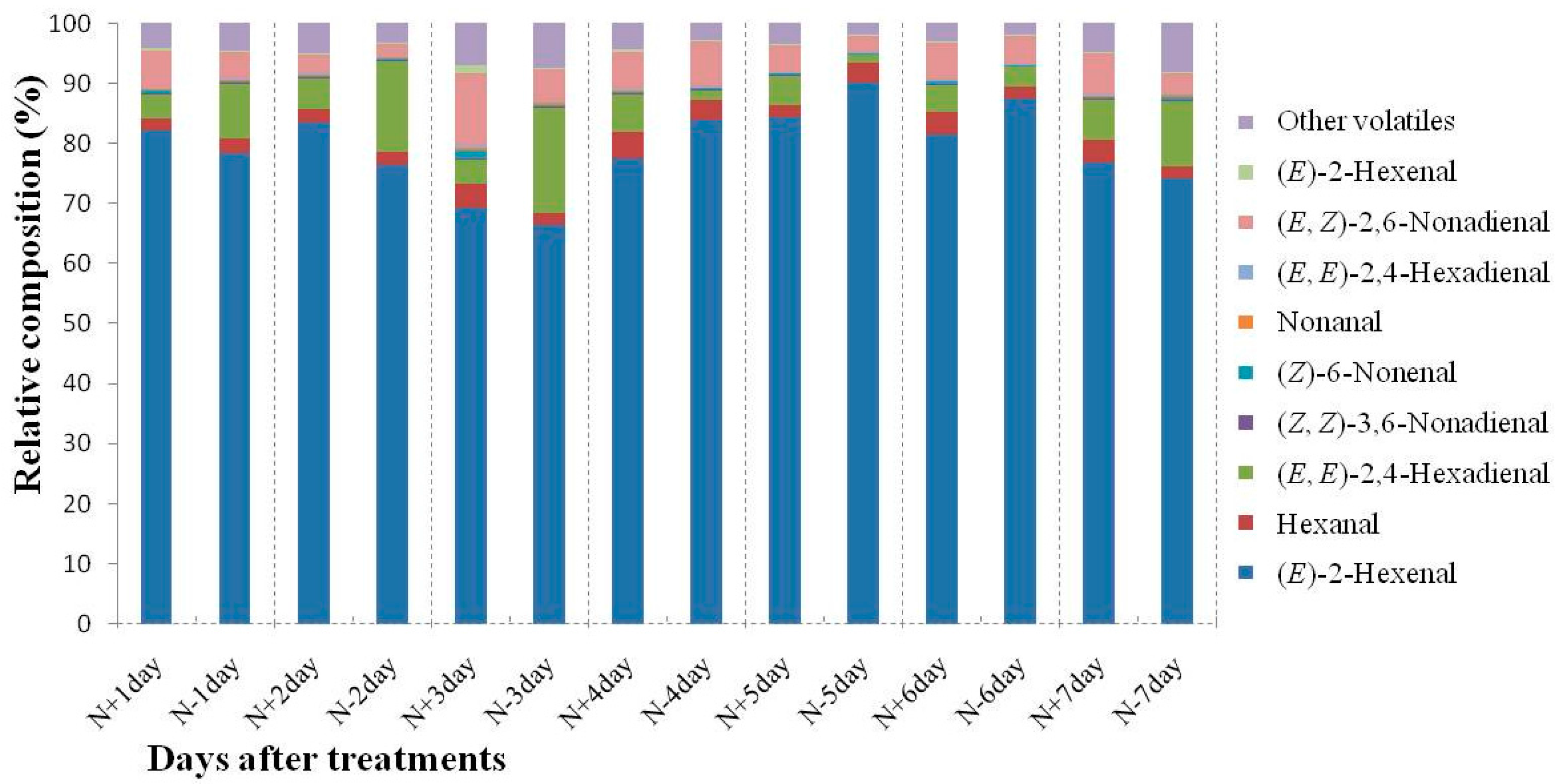
2.1. Analysis of Cucumber Leaf Volatiles by GC-MS and Variation in Total Volatile Content
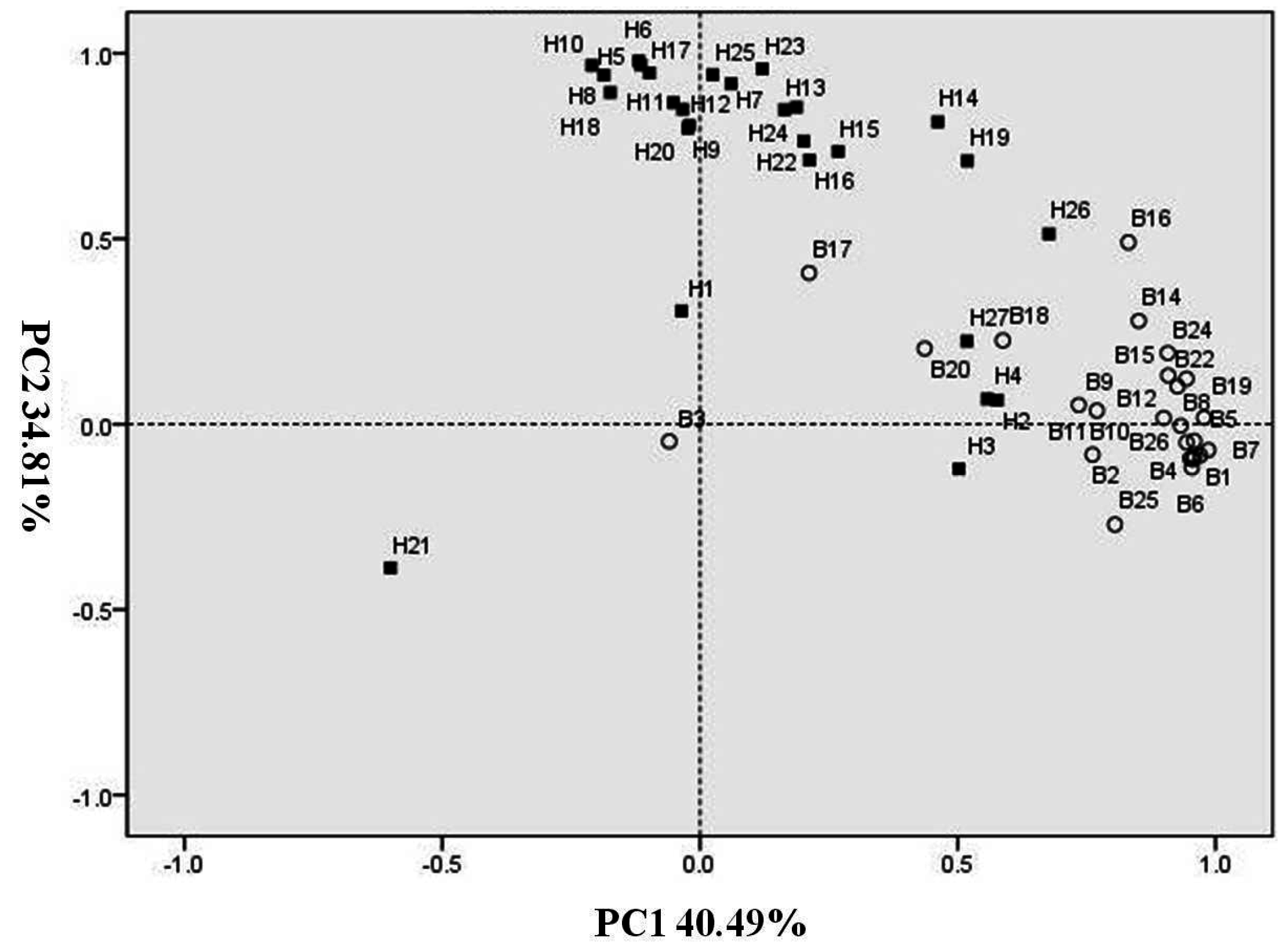
2.2. Principal Component Analysis of Volatiles during N-Sufficient and N-Deficient Treatments
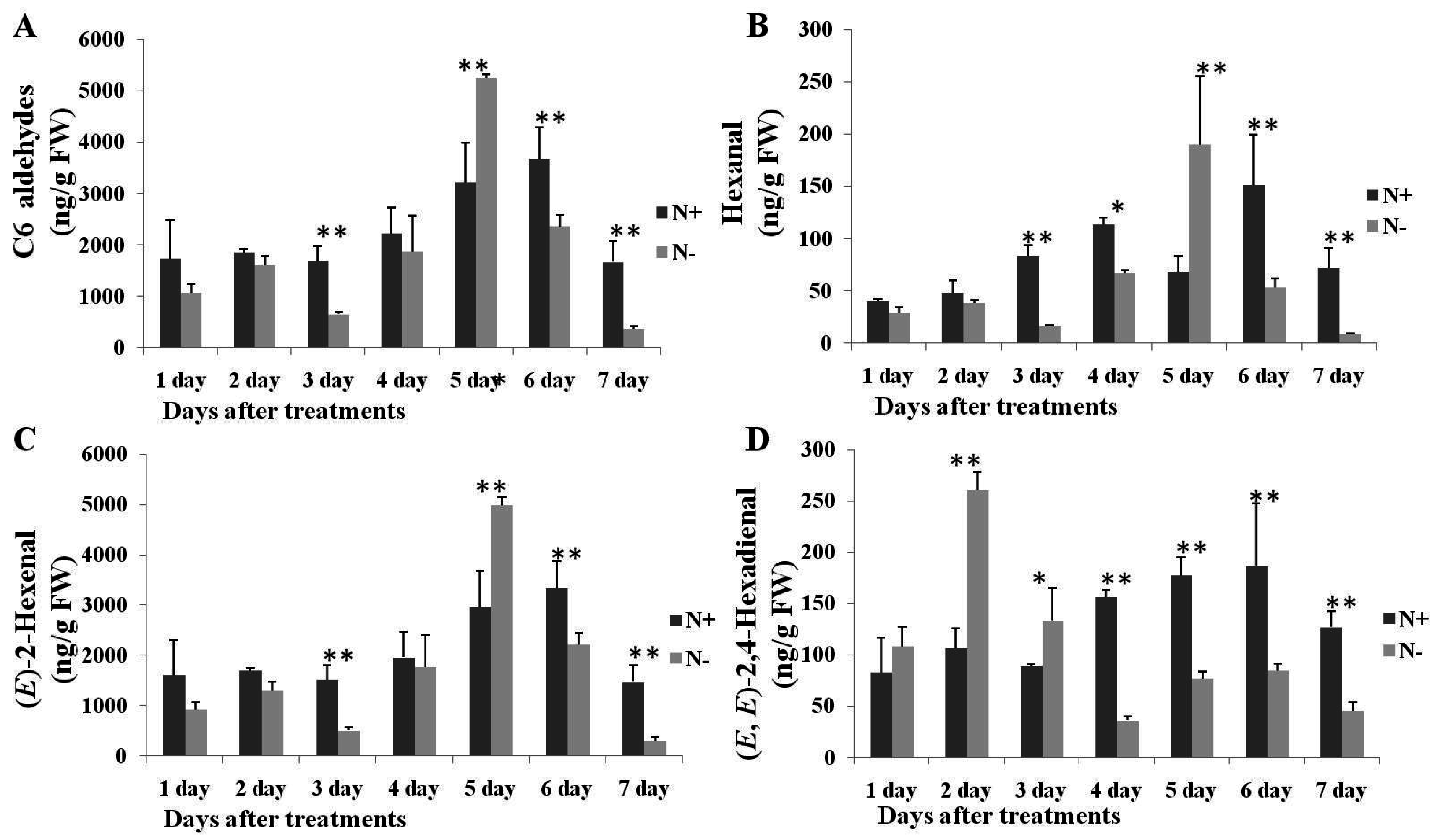
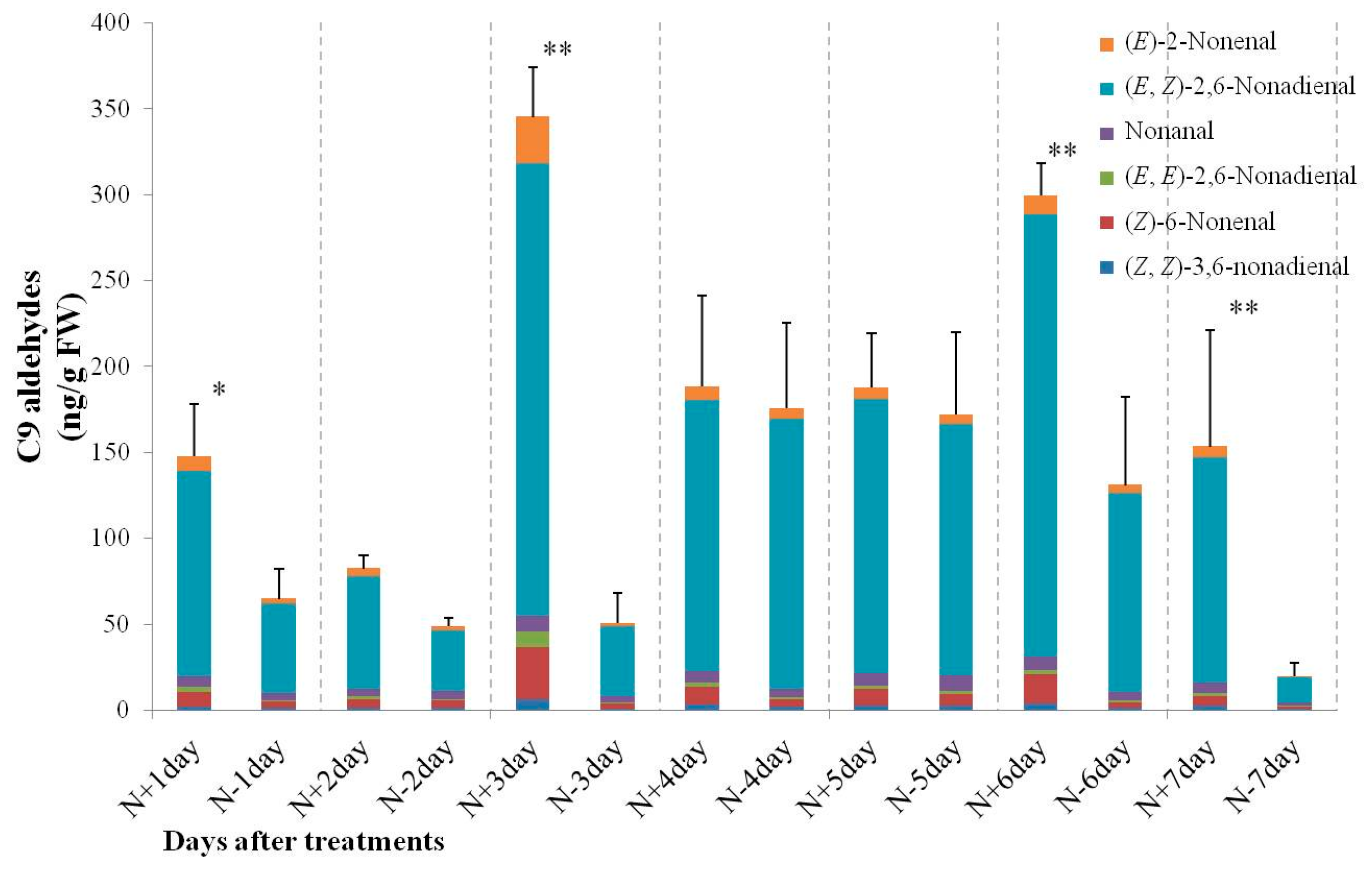
2.3. Changes of C6 and C9 Aldehyde Content during N-Deficient Treatment
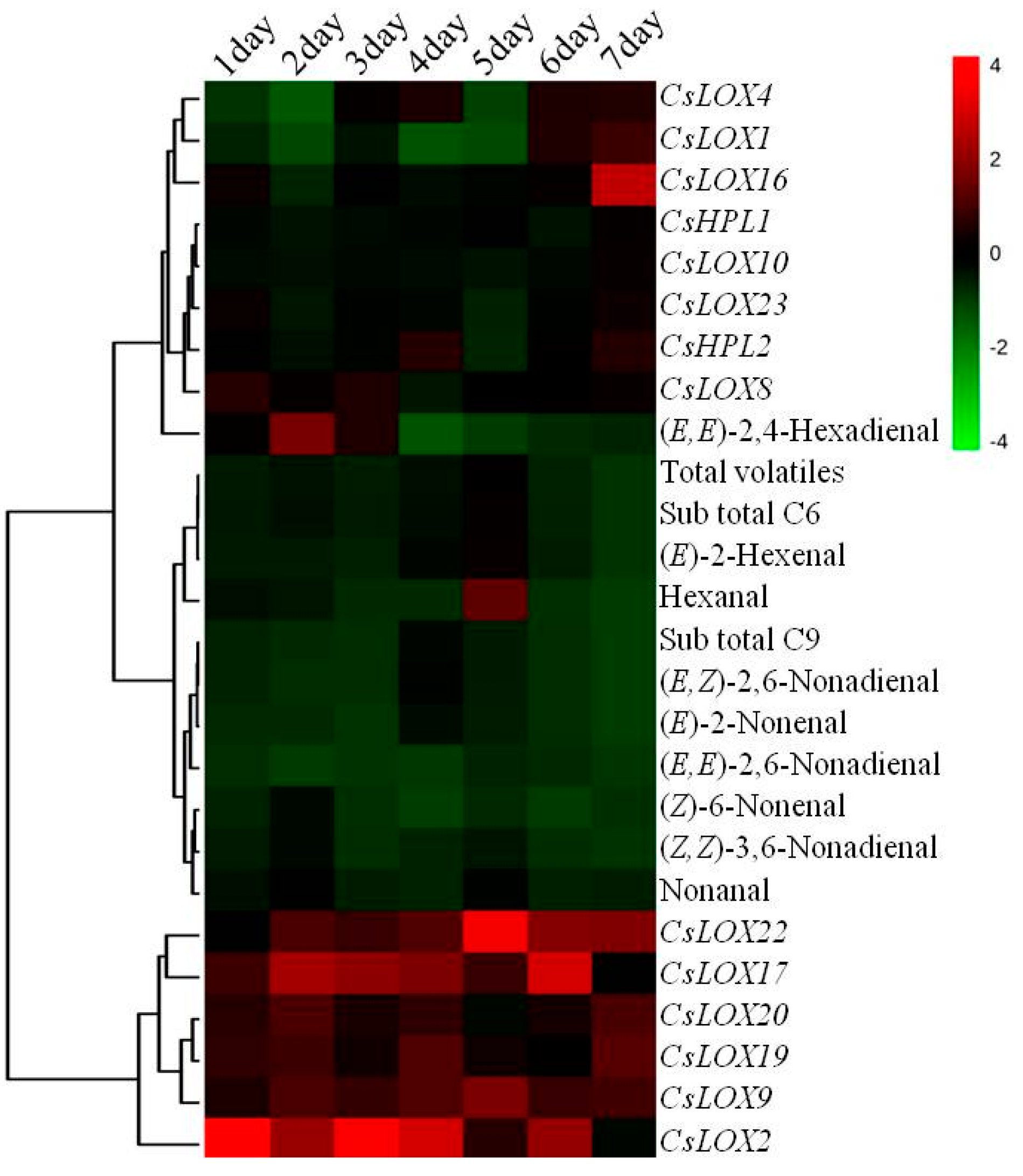
2.4. Relationship between C6 and C9 Aldehydes and Gene Expressions in the 9930 Line
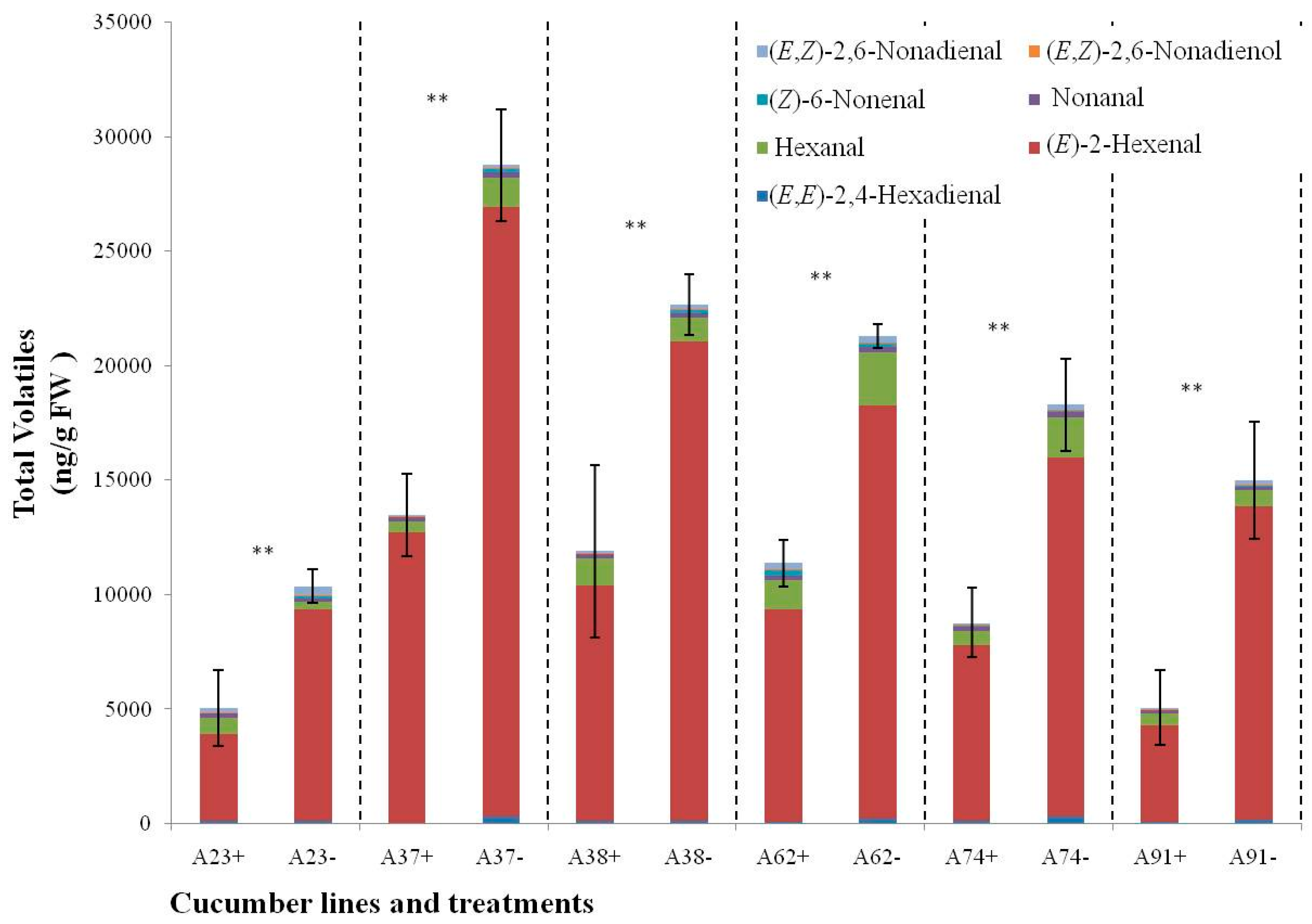
2.5. Changes of C6 and C9 Volatile Content during N-Deficient Treatment in Six Other Inbred Cucumber Lines
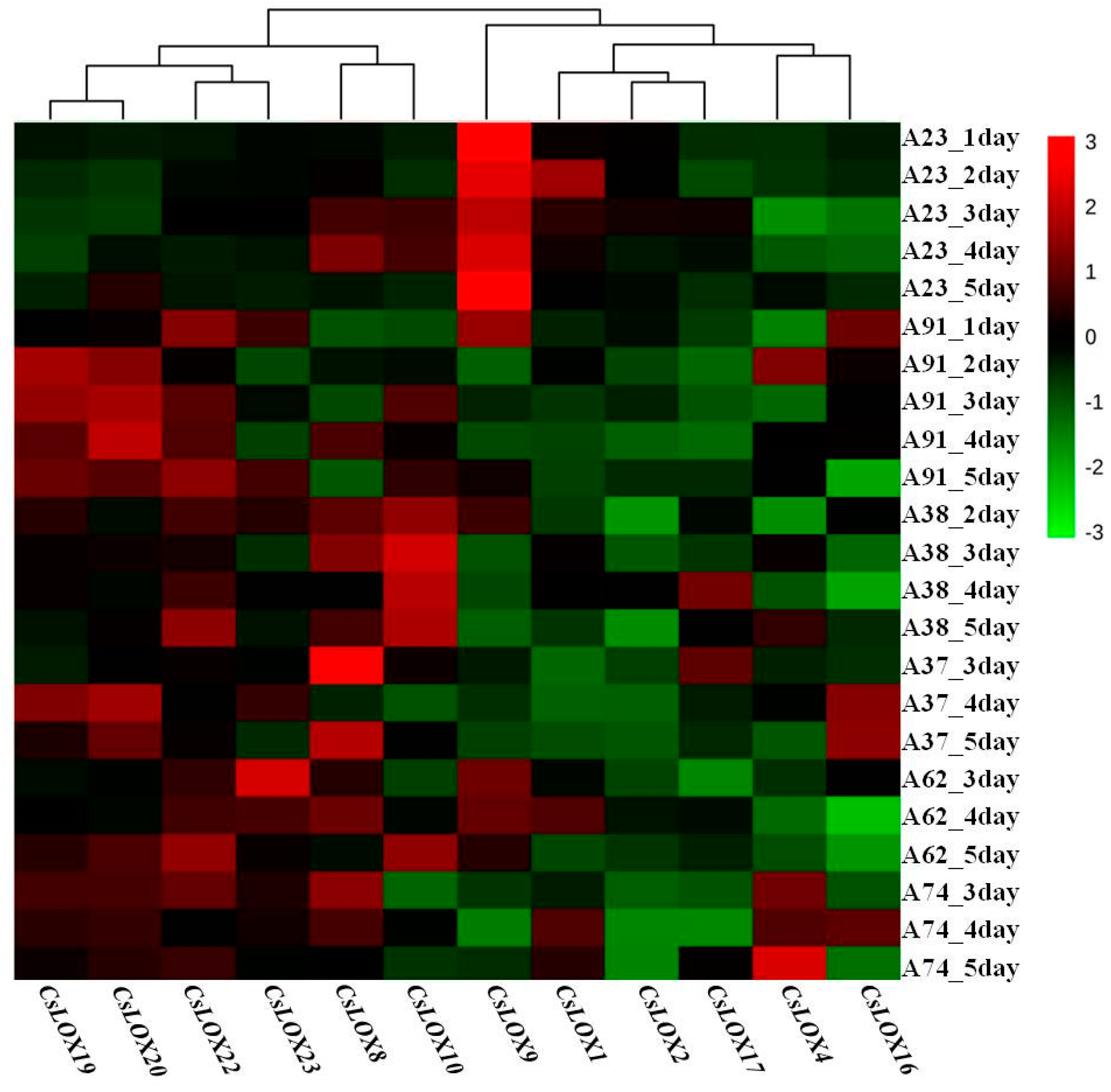
2.6. The Expression Patterns of Cucumber LOX Genes in Response to N-Deficient Treatments in Six Inbred Cucumber Lines
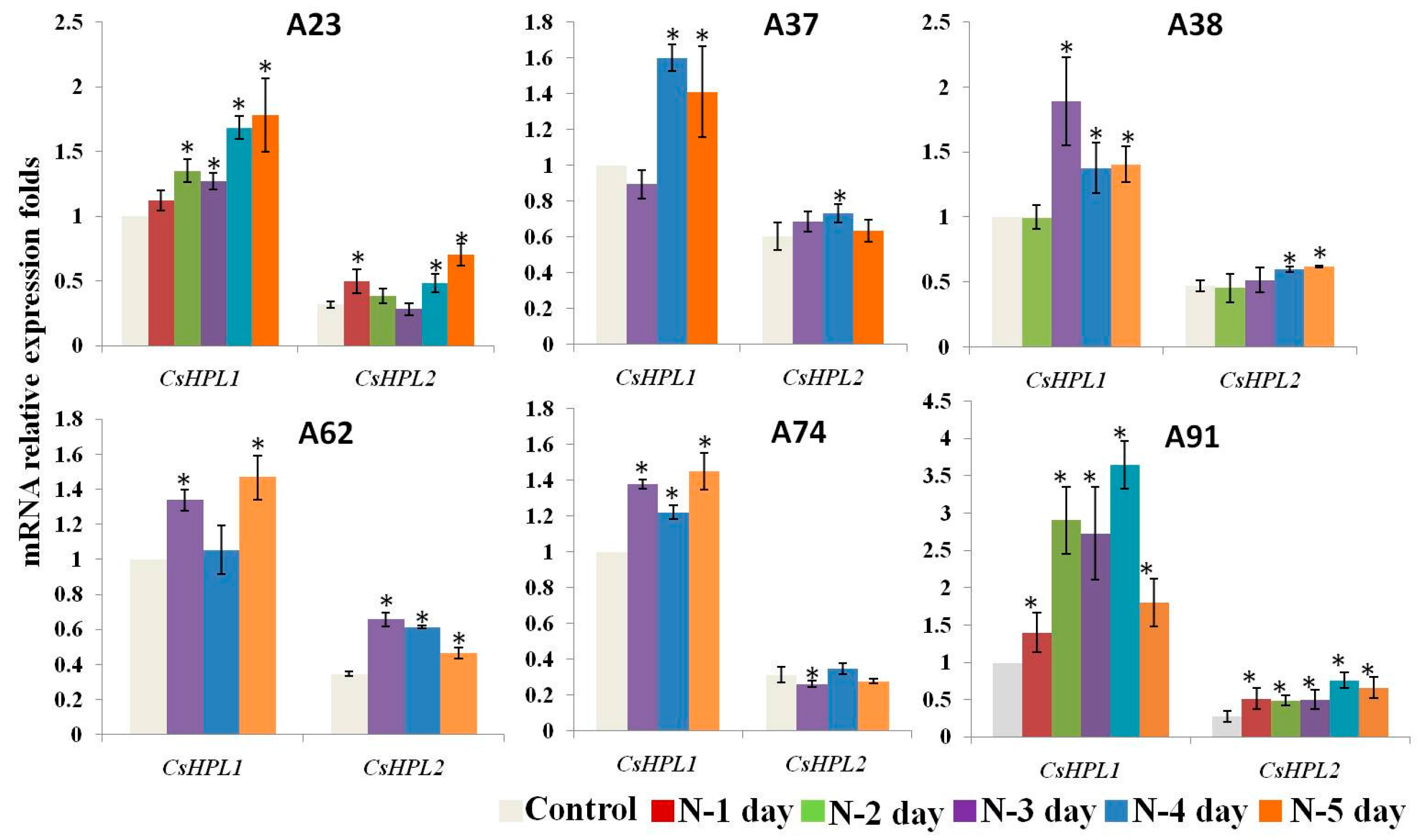
2.7. The Expression Patterns of CsHPLs during N-Deficient Treatments in Six Inbred Cucumber Lines
3. Material and Methods
3.1. Plant Materials and N-Deficient Treatments
3.2. HS-SPME Extraction
3.3. GC-MS Analysis
3.4. RNA Isolation and qPCR
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Palma-Harris, C.; McFeeters, R.F.; Fleming, H.P. Solid-phase microextraction (SPME) technique for measurement of generation of fresh cucumber flavor compounds. J. Agric. Food Chem. 2001, 49, 4203–4207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Diamond, J. China’s environment in a globalizing world. Nature 2005, 435, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Yang, X.Y.; Yu, H.J.; Jiang, W.J.; Sun, N.; Liu, X.R.; Liu, X.L.; Zhang, X.M.; Wang, Y.; Gu, X.F. RNA-Seq-Based Transcriptome Profiling of Early Nitrogen Deficiency Response in Cucumber Seedlings Provides New Insight into the Putative Nitrogen Regulatory Network. Plant Cell Physiol. 2015, 56, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Huang, S.W.; Liu, R.L.; Jin, J.Y. Effects of nitrogen application on flavor compounds of cherry tomato fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Hoffland, E.; van Beusichem, M.L.; Jeger, M.J. Nitrogen availability and susceptibility of tomato leaves to Botrytis cinerea. Plant Soil 1999, 210, 263–272. [Google Scholar] [CrossRef]
- Zafar, Z.U.; Athar, H.U.R.; Ashraf, M. Responses of Two Cotton (Gossypium hirsutum L.) Cultivars Differing in Resistance to Leaf Curl Virus Disease to Nitrogen Nutrition. Pak. J. Bot. 2010, 42, 2085–2094. [Google Scholar]
- Zhang, X.; Yu, H.J.; Zhang, X.M.; Yang, X.Y.; Zhao, W.C.; Li, Q.; Jiang, W.J. Effect of nitrogen deficiency on ascorbic acid biosynthesis and recycling pathway in cucumber seedlings. Plant Physiol. Biochem. 2016, 108, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, R.; Hao, L.; Chen, W.; Cheng, S. Profiling of volatile compounds and associated gene expression and enzyme activity during fruit development in two cucumber cultivars. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Siangdung, W.; Fukushige, H.; Hildebrand, D. Hydroperoxidelyase and leaf aldehyde formation can be greatly increased in leaves. Adv. Res. Plant Lipids 2003. [Google Scholar] [CrossRef]
- Scala, A.; Mirabella, R.; Mugo, C.; Matsui, K.; Haring, M.A.; Schuurink, R.C. E-2-hexenal promotes susceptibility to Pseudomonas syringae by activating jasmonic acid pathways in Arabidopsis. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Jiang, W.J.; Yu, H.J. The Expression Profiling of the Lipoxygenase (LOX) Family Genes during Fruit Development, Abiotic Stress and Hormonal Treatments in Cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2012, 13, 2481–2500. [Google Scholar] [CrossRef] [PubMed]
- Forss, D.; Dunstone, E.; Ramshaw, E.; Stark, W. The flavor of cucumbers. J. Food Sci. 1962, 27, 90–93. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J.; Furstenau, B. Emission of herbivore-induced volatiles in absence of a herbivore—Response of Zea mays to green leaf volatiles and terpenoids. Z. Naturforsch. C. 2005, 60, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J.; Kleier, S. Plant-plant signaling: Ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-Hexen-1-ol. J. Chem. Ecol. 2005, 31, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.; Juttner, F.; Slusarenko, A.J. Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae pv. phaseolicola. Plant Physiol. 1993, 101, 13–24. [Google Scholar] [PubMed]
- Shiojiri, K.; Ozawa, R.; Matsui, K.; Kishimoto, K.; Kugimiya, S.; Takabayashi, J. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 1996, 32, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Heiden, A.C.; Kobel, K.; Langebartels, C.; Schuh-Thomas, G.; Wildt, J. Emissions of oxygenated volatile organic compounds from plants—Part I: Emissions from lipoxygenase activity. J. Atmos. Chem. 2003, 45, 143–172. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F. Fatty acid hydroperoxidelyase: A plant cytochrome p450 enzyme involved in wound healing and pest resistance. Chembiochem 2001, 2, 494–504. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.H.; Chen, S.X.; Wang, C.Y.; Zhang, R.R.; Cheng, S.Q. Isolation, expression, and characterization of a hydroperoxidelyase gene from cucumber. Int. J. Mol. Sci. 2013, 14, 22082–22101. [Google Scholar] [CrossRef] [PubMed]
- Liavonchanka, A.; Feussner, N. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Ujita, C.; Fujimoto, S.; Wilkinson, J.; Hiatt, B.; Knauf, V.; Kajiwara, T.; Feussner, I. Fatty acid 9- and 13-hydroperoxide lyases from cucumber. FEBS Lett. 2000, 481, 183–188. [Google Scholar] [CrossRef]
- Buescher, R.H.; Buescher, R.W. Production and stability of (E,Z)-2,6-nonadienal, the major flavor volatile of cucumbers. J. Food. Sci. 2001, 66, 357–361. [Google Scholar] [CrossRef]
- Liu, S.Q.; Liu, X.H.; Jiang, L.W. Genome-wide identification, phylogeny and expression analysis of the lipoxygenase gene family in cucumber. Genet. Mol. Res. 2011, 10, 2613–2636. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Jiang, Q.; Zhang, B.; Xu, C.J.; Chen, K.S. Citrus Leaf Volatiles as Affected by Developmental Stage and Genetic Type. Int. J. Mol. Sci. 2013, 14, 17744–17766. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, 251–257. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Yu, H.-J.; Li, Y.-Y.; Zhang, X.-M.; Liu, P.; Li, Q.; Jiang, W.-J. Leaf Volatile Compounds and Associated Gene Expression during Short-Term Nitrogen Deficient Treatments in Cucumis Seedlings. Int. J. Mol. Sci. 2016, 17, 1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111713
Deng J, Yu H-J, Li Y-Y, Zhang X-M, Liu P, Li Q, Jiang W-J. Leaf Volatile Compounds and Associated Gene Expression during Short-Term Nitrogen Deficient Treatments in Cucumis Seedlings. International Journal of Molecular Sciences. 2016; 17(11):1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111713
Chicago/Turabian StyleDeng, Jie, Hong-Jun Yu, Yun-Yun Li, Xiao-Meng Zhang, Peng Liu, Qiang Li, and Wei-Jie Jiang. 2016. "Leaf Volatile Compounds and Associated Gene Expression during Short-Term Nitrogen Deficient Treatments in Cucumis Seedlings" International Journal of Molecular Sciences 17, no. 11: 1713. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111713





