The Activating NKG2C Receptor Is Significantly Reduced in NK Cells after Allogeneic Stem Cell Transplantation in Patients with Severe Graft-versus-Host Disease
Abstract
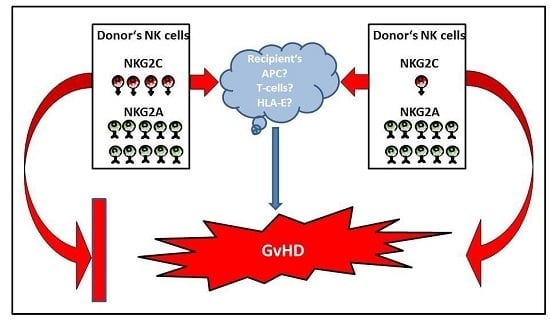
:1. Introduction
2. Results
2.1. Reduced Proportion of NK Cells Expressing the Activating CD94/NKG2C Receptor Pair in Patients with Severe Acute or Chronic GvHD
2.2. Reduced Proportions of NK Cells Expressing the Inhibitory CD94/NKG2A Receptor Pair in Patients with Extended Chronic GvHD after HLA-Identical AlloSCT
2.3. Reduced Ratio of CD94/NKG2C to CD94/NKG2A in Patients with Severe Acute or Chronic GvHD after HLA-Mismatched AlloSCT
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farag, S.S.; Fehniger, T.A.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cell receptors: New biology and insights into the graft-versus-leukemia effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.T.; Schaffer, M.; Fauriat, C.; Ringden, O.; Remberger, M.; Hammarstedt, C.; Barrett, A.J.; Ljungman, P.; Ljunggren, H.G.; Malmberg, K.J. NK cells expressing inhibitory KIR for non-self-ligands remain tolerant in HLA-matched sibling stem cell transplantation. Blood 2010, 115, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Dhedin, N.; Vernant, J.P.; Kuentz, M.; Al Jijakli, A.; Rouas-Freiss, N.; Carosella, E.D.; Boudifa, A.; Debre, P.; Vieillard, V. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: Immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005, 105, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, N.K.; Riese, P.; Heuts, F.; Andersson, S.; Fauriat, C.; Ivarsson, M.A.; Bjorklund, A.T.; Flodstrom-Tullberg, M.; Michaelsson, J.; Rottenberg, M.E.; et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010, 116, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Shilling, H.G.; McQueen, K.L.; Cheng, N.W.; Shizuru, J.A.; Negrin, R.S.; Parham, P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood 2003, 101, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Botet, M.; Angulo, A.; Guma, M. Natural killer cell receptors for major histocompatibility complex class I and related molecules in cytomegalovirus infection. Tissue Antigens 2004, 63, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lazetic, S.; Chang, C.; Houchins, J.P.; Lanier, L.L.; Phillips, J.H. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J. Immunol. 1996, 157, 4741–4745. [Google Scholar] [PubMed]
- Kaiser, B.K.; Barahmand-Pour, F.; Paulsene, W.; Medley, S.; Geraghty, D.E.; Strong, R.K. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J. Immunol. 2005, 174, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.; Jones, E.Y.; McMichael, A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 1997, 27, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Bland, F.A.; Lemberg, M.K.; McMichael, A.J.; Martoglio, B.; Braud, V.M. Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E. J. Biol. Chem. 2003, 278, 33747–33752. [Google Scholar] [CrossRef] [PubMed]
- Hoare, H.L.; Sullivan, L.C.; Clements, C.S.; Ely, L.K.; Beddoe, T.; Henderson, K.N.; Lin, J.; Reid, H.H.; Brooks, A.G.; Rossjohn, J. Subtle changes in peptide conformation profoundly affect recognition of the non-classical MHC class I molecule HLA-E by the CD94-NKG2 natural killer cell receptors. J. Mol. Biol. 2008, 377, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Karre, K. Immunology. A perfect mismatch. Science 2002, 295, 2029–2031. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Mancusi, A.; Burchielli, E.; Perruccio, K.; Aversa, F.; Martelli, M.F.; Velardi, A. Natural killer cell recognition of missing self and haploidentical hematopoietic transplantation. Semin. Cancer Biol. 2006, 16, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Pende, D.; Mingari, M.C.; Bertaina, A.; Falco, M.; Moretta, A.; Moretta, L. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: Role of alloreactive NK cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.T.; Clancy, T.; Goodridge, J.P.; Beziat, V.; Schaffer, M.; Hovig, E.; Ljunggren, H.G.; Ljungman, P.T.; Malmberg, K.J. Naive Donor NK Cell Repertoires Associated with Less Leukemia Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. J. Immunol. 2016, 196, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J.; et al. CD56dimCD57 + NKG2C + NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Picardi, A.; Mengarelli, A.; Marino, M.; Gallo, E.; Benevolo, M.; Pescarmona, E.; Cocco, R.; Fraioli, R.; Tremante, E.; Petti, M.C.; et al. Up-regulation of activating and inhibitory NKG2 receptors in allogeneic and autologous hematopoietic stem cell grafts. J. Exp. Clin. Cancer Res. 2015, 34. [Google Scholar] [CrossRef] [PubMed]
- Kheav, V.D.; Busson, M.; Scieux, C.; Peffault de Latour, R.; Maki, G.; Haas, P.; Mazeron, M.C.; Carmagnat, M.; Masson, E.; Xhaard, A.; et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica 2014, 99, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Wieten, L.; Mahaweni, N.M.; Voorter, C.E.; Bos, G.M.; Tilanus, M.G. Clinical and immunological significance of HLA-E in stem cell transplantation and cancer. Tissue Antigens 2014, 84, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Danzer, M.; Polin, H.; Proll, J.; Haunschmid, R.; Hofer, K.; Stabentheiner, S.; Hackl, C.; Kasparu, H.; Konig, J.; Hauser, H.; et al. Clinical significance of HLA-E*0103 homozygosity on survival after allogeneic hematopoietic stem-cell transplantation. Transplantation 2009, 88, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Tamouza, R.; Busson, M.; Rocha, V.; Fortier, C.; Haddad, Y.; Brun, M.; Boukouaci, W.; Bleux, H.; Socie, G.; Krishnamoorthy, R.; et al. Homozygous status for HLA-E*0103 confers protection from acute graft-versus-host disease and transplant-related mortality in HLA-matched sibling hematopoietic stem cell transplantation. Transplantation 2006, 82, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Ludajic, K.; Rosenmayr, A.; Fae, I.; Fischer, G.F.; Balavarca, Y.; Bickeboller, H.; Kalhs, P.; Greinix, H.T. Association of HLA-E polymorphism with the outcome of hematopoietic stem-cell transplantation with unrelated donors. Transplantation 2009, 88, 1227–1228. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar] [PubMed]



| Characteristics | All | HLA-Identical AlloSCT | HLA-Mismatched AlloSCT |
|---|---|---|---|
| Number of patients | 26 | 14 | 12 |
| Median age (years (range)) | 51 (21–69) | 51 (23–69) | 47 (21–69) |
| Gender (female/male) | 17/9 | 9/5 | 8/4 |
| Diagnosis at alloSCT | |||
| AML | 20 | 11 | 9 |
| sAML | 4 | 3 | 1 |
| MDS | 1 | 0 | 1 |
| T-NHL | 1 | 0 | 1 |
| Gender mismatch | 13 | 9 | 4 |
| Follow-up time after alloSCT (median days (range)) | 1570 (55–2004) | 1570 (435–1798) | 1553 (55–2004) |
| GvHD | |||
| acute GvHD grade 0-I | 16 | 9 | 7 |
| acute GvHD grade II-IV | 10 | 5 | 5 |
| no/limited chronic GvHD * | 13 | 7 | 6 |
| extended chronic GvHD * | 10 | 7 | 3 |
| Relapse ** | 3 | 2 | 1 |
| Alive/dead ** | 19/7 | 12/2 | 7/5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordelas, L.; Steckel, N.-K.; Horn, P.A.; Beelen, D.W.; Rebmann, V. The Activating NKG2C Receptor Is Significantly Reduced in NK Cells after Allogeneic Stem Cell Transplantation in Patients with Severe Graft-versus-Host Disease. Int. J. Mol. Sci. 2016, 17, 1797. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111797
Kordelas L, Steckel N-K, Horn PA, Beelen DW, Rebmann V. The Activating NKG2C Receptor Is Significantly Reduced in NK Cells after Allogeneic Stem Cell Transplantation in Patients with Severe Graft-versus-Host Disease. International Journal of Molecular Sciences. 2016; 17(11):1797. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111797
Chicago/Turabian StyleKordelas, Lambros, Nina-Kristin Steckel, Peter A. Horn, Dietrich W. Beelen, and Vera Rebmann. 2016. "The Activating NKG2C Receptor Is Significantly Reduced in NK Cells after Allogeneic Stem Cell Transplantation in Patients with Severe Graft-versus-Host Disease" International Journal of Molecular Sciences 17, no. 11: 1797. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111797