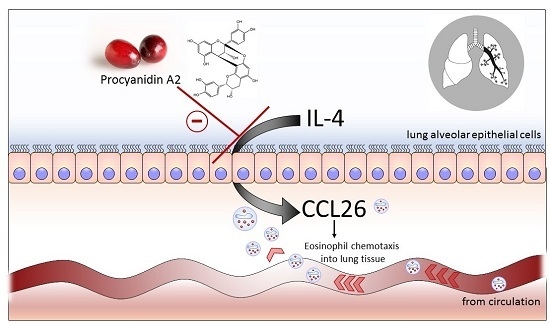
Procyanidin A2 Modulates IL-4-Induced CCL26 Production in Human Alveolar Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Optimization of Airway Epithelial Cell Bioassay Conditions
2.2. Cytotoxicity Assessment
2.3. Evaluation of Procyanidin A2
2.4. Time-Dependent Inhibition of CCL26 (Eotaxin-3) by Procyanidin A2 and IFNγ (Interferon γ)
2.5. Procyanidin A2 Impedes IFNγ-Mediated CCL26 Inhibition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture Conditions
4.3. Optimizing the Production of CCL26
4.4. Procyanidin Preparation
4.5. Cytotoxicity
4.6. Modulation of CCL26
4.7. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCL26 | eotaxin-3 |
| DMEM/F-12 | Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 |
| FBS | fetal bovine serum |
| IFNγ | interferon γ |
| LDH | lactate dehydrogenase |
| MAPK | mitogen-activated protein kinases |
| NADH | β-nicotinamide adenine dinucleotide reduced dipotassium salt |
| NK-κB | nuclear factor κ-light-chain-enhancer of activated B cells |
| STAT | signal transducers and activators of transcription |
| USDA | United States Department of Agriculture |
| WST-1 | water soluble tetrazolium-1 |
References
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menendez, J.A.; Bioactive Food Components Platform. Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Ardevol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.L.; Hurst, R.D.; Sawyer, G.M.; Kruger, M.C. Fruit Procyanidins: Modulating Inflammation to Promote Health. In Proanthocyanidins: Food Sources, Antioxidant Properties, and Health Benefits; Sullivan, I., Ed.; Nova Science: New York, NY, USA, 2015; pp. 73–97. [Google Scholar]
- Usual Dietary Intakes: Food Intakes, U.S. Population, 2007-10. Available online: http://epi.grants.cancer.gov/diet/usualintakes/pop/2007-10/ (accessed on 2 February 2016).
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Nyanhanda, T.; Gould, E.M.; Hurst, R.D. Plant-derived foods for the attenuation of allergic airway inflammation. Curr. Pharm. Des. 2014, 20, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Ishmael, F.T. The inflammatory response in the pathogenesis of asthma. J. Am. Osteopath. Assoc. 2011, 111, S11–S17. [Google Scholar] [PubMed]
- Hallstrand, T.S.; Hackett, T.L.; Altemeier, W.A.; Matute-Bello, G.; Hansbro, P.M.; Knight, D.A. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin. Immunol. 2014, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Skurkovich, S.; Skurkovich, B. Anticytokine therapy, especially anti-interferon-γ, as a pathogenetic treatment in Th-1 autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.H.; Maher, S.G.; Young, H.A. Clinical use of interferon-γ. Ann. N. Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Heller, N.M.; Matsukura, S.; Georas, S.N.; Boothby, M.R.; Rothman, P.B.; Stellato, C.; Schleimer, R.P. Interferon-γ inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004, 31, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Boguniewicz, M.; Henson, J.E.; Celniker, A.C.; Williams, M.; Giorno, R.C.; Leung, D.Y.M. The effects of inhaled interferon-γ in normal human airways. Am. Rev. Respir. Dis. 1993, 148, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Flaishon, L.; Topilski, I.; Shoseyov, D.; Hershkoviz, R.; Fireman, E.; Levo, Y.; Marmor, S.; Shachar, I. Cutting edge: Anti-inflammatory properties of low levels of IFN-γ. J. Immunol. 2002, 168, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kobayashi, I.; Tsuji, K.; Nishi, N.; Muro, E.; Miyazaki, M.; Zaitsu, M.; Inada, S.; Ichimaru, T.; Hamasaki, Y. Upregulation of interieukin-4 receptor by interferon-γ—Enhanced interleukin-4-induced eotaxin-3 production in airway epithelium. Am. J. Respir. Cell Mol. Biol. 2004, 31, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Provost, K.; Niu, N.; Homer, R.; Cohn, L. IFN-γ acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. J. Immunol. 2011, 187, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.-P.; Salvado, M.-J.; Bustos, M.; Fernandez-Larrea, J.; Motilva, M.-J. Determination of procyanidins and their metabolites in plasma samples by improved liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2009, 877, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.L.; Hurst, R.D.; Sawyer, G.M.; Kruger, M.C. The in vitro evaluation of isolated procyanidins as modulators of cytokine-induced eotaxin production in human alveolar epithelial cells. J. Berry Res. 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Pierini, R.; Kroon, P.A.; Guyot, S.; Ivory, K.; Johnson, I.T.; Belshaw, N.J. Procyanidin effects on oesophageal adenocarcinoma cells strongly depend on flavan-3-ol degree of polymerization. Mol. Nutr. Food Res. 2008, 52, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.G.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Dimeric procyanidins are inhibitors of NF-κB-DNA binding. Biochem. Pharmacol. 2009, 78, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Godot, V.; Humbert, M. New chemokine targets for asthma therapy. Curr. Allergy Asthma Rep. 2005, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kawate, T.; Liu, X.; Kim, Y.B.; Zhao, Y.; Feng, G.; Banerji, J.; Nash, H.; Whitehurst, C.; Jindal, S.; et al. STAT6 phosphorylation inhibitors block eotaxin-3 secretion in bronchial epithelial cells. Bioorg. Med. Chem. 2012, 20, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Hebenstreit, D.; Luft, P.; Schmiedlechner, A.; Duschl, A.; Horejs-Hoeck, J. SOCS-1 and SOCS-3 inhibit IL-4 and IL-13 induced activation of Eotaxin-3/CCL26 gene expression in HEK293 cells. Mol. Immunol. 2005, 42, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Rothenberg, M.E. Demethylation of the human eotaxin-3 gene promoter leads to the elevated expression of eotaxin-3. J. Immunol. 2014, 192, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic. Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Tanabe, S.; Howell, A.; Grenier, D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Chou, M.Y.; Howell, A.; Wobbe, C.; Grady, R.; Stapleton, A.E. Cranberry products inhibit adherence of P-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J. Urol. 2007, 177, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Povoas, M.J.; Mateus, N.; de Freitas, V. Application of flow nephelometry to the analysis of the influence of carbohydrates on protein-tannin interactions. J. Sci. Food Agric. 2006, 86, 891–896. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. Interactions of flavan-3-ols and procyanidins with membranes: Mechanisms and the physiological relevance. Food Funct. 2015, 6, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, E.J.M. Environmental influences on signal transduction through membranes: A retrospective mini-review. Biophys. Chem. 2003, 100, 519–534. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Hammerstone, J.F.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J. Agric. Food Chem. 2005, 53, 5041–5048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiong, L.; Peng, J.M.; Deng, X.Y.; Gao, J.; Li, C.M. Structure-dependent membrane-perturbing potency of four proanthocyanidin dimers on 3T3-L1 preadipocytes. J. Agric. Food Chem. 2016, 64, 7022–7032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zou, B.; Nie, R.Z.; Zhang, Y.; Li, C.M. A-type ECG and EGCG dimers disturb the structure of 3T3-L1 cell membrane and strongly inhibit its differentiation by targeting peroxisome proliferator-activated receptor γ with miR-27 involved mechanism. J. Nur. Biochem. 2015, 26, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.M.; McGhie, T.K.; Cooney, J.M.; Jensen, D.J.; Gould, E.M.; Lyall, K.A.; Hurst, R.D. Blackcurrant proanthocyanidins augment IFN-γ-induced suppression of IL-4 stimulated CCL26 secretion in alveolar epithelial cells. Mol. Nutr. Food Res. 2010, 54, S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Nyanhanda, T.; Gould, E.M.; McGhie, T.; Shaw, O.M.; Harper, J.L.; Hurst, R.D. Blackcurrant cultivar polyphenolic extracts suppress CCL26 secretion from alveolar epithelial cells. Food Funct. 2014, 5, 671–677. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, S.L.; Kruger, M.C.; Sawyer, G.M.; Hurst, R.D. Procyanidin A2 Modulates IL-4-Induced CCL26 Production in Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2016, 17, 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111888
Coleman SL, Kruger MC, Sawyer GM, Hurst RD. Procyanidin A2 Modulates IL-4-Induced CCL26 Production in Human Alveolar Epithelial Cells. International Journal of Molecular Sciences. 2016; 17(11):1888. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111888
Chicago/Turabian StyleColeman, Sara L., Marlena C. Kruger, Gregory M. Sawyer, and Roger D. Hurst. 2016. "Procyanidin A2 Modulates IL-4-Induced CCL26 Production in Human Alveolar Epithelial Cells" International Journal of Molecular Sciences 17, no. 11: 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111888