Hydrostatin-TL1, an Anti-Inflammatory Active Peptide from the Venom Gland of Hydrophis cyanocinctus in the South China Sea
Abstract
:1. Introduction
2. Results and Discussion
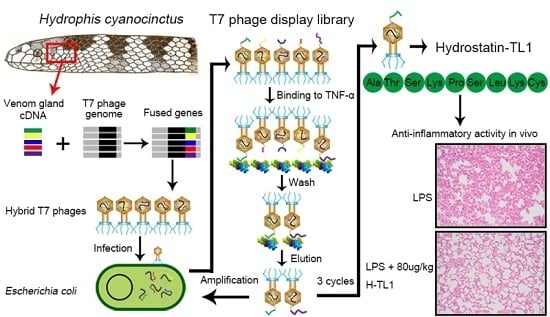
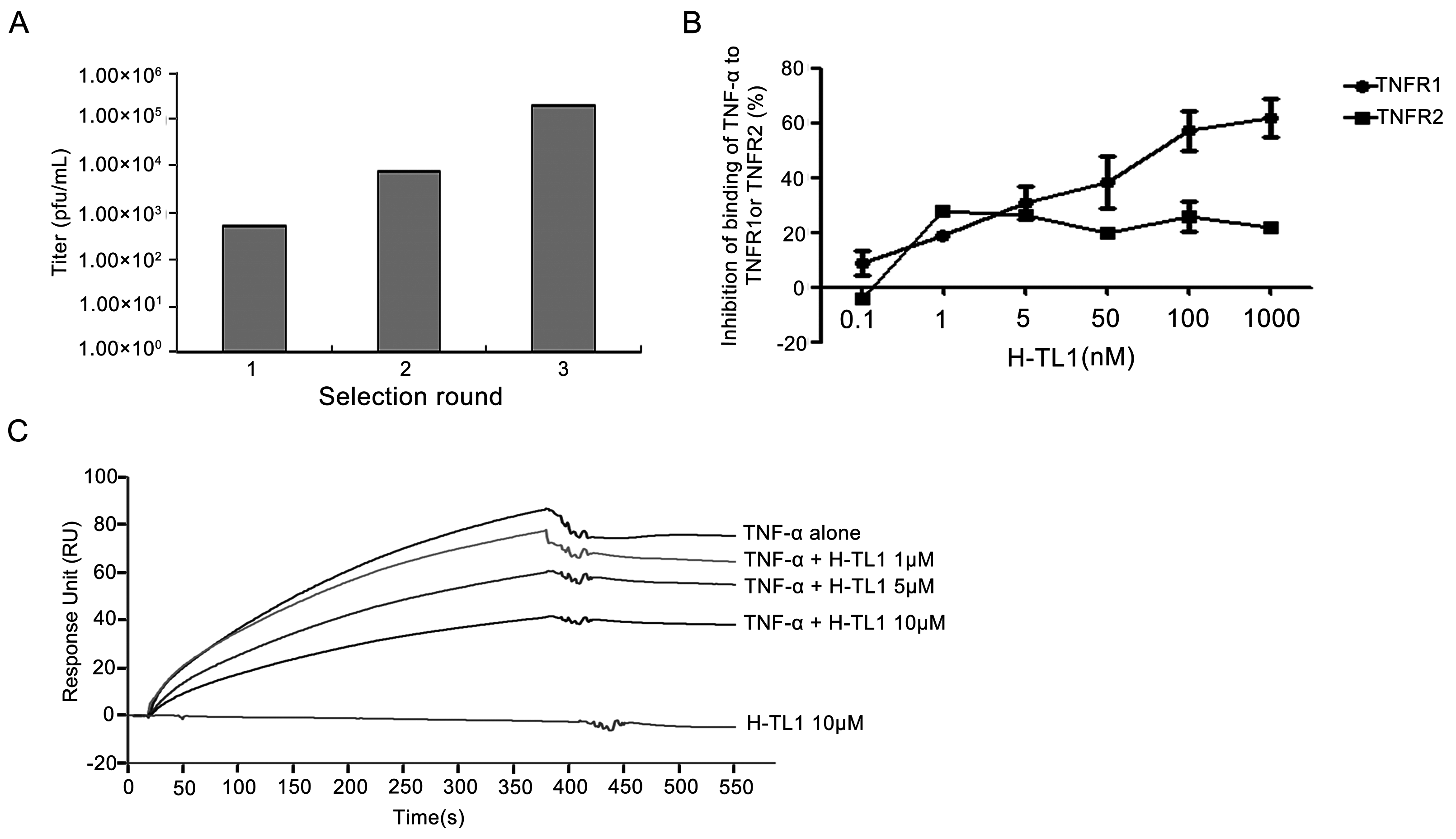
2.1. Biopanning and Sequencing
2.2. Competitive Inhibition Assays
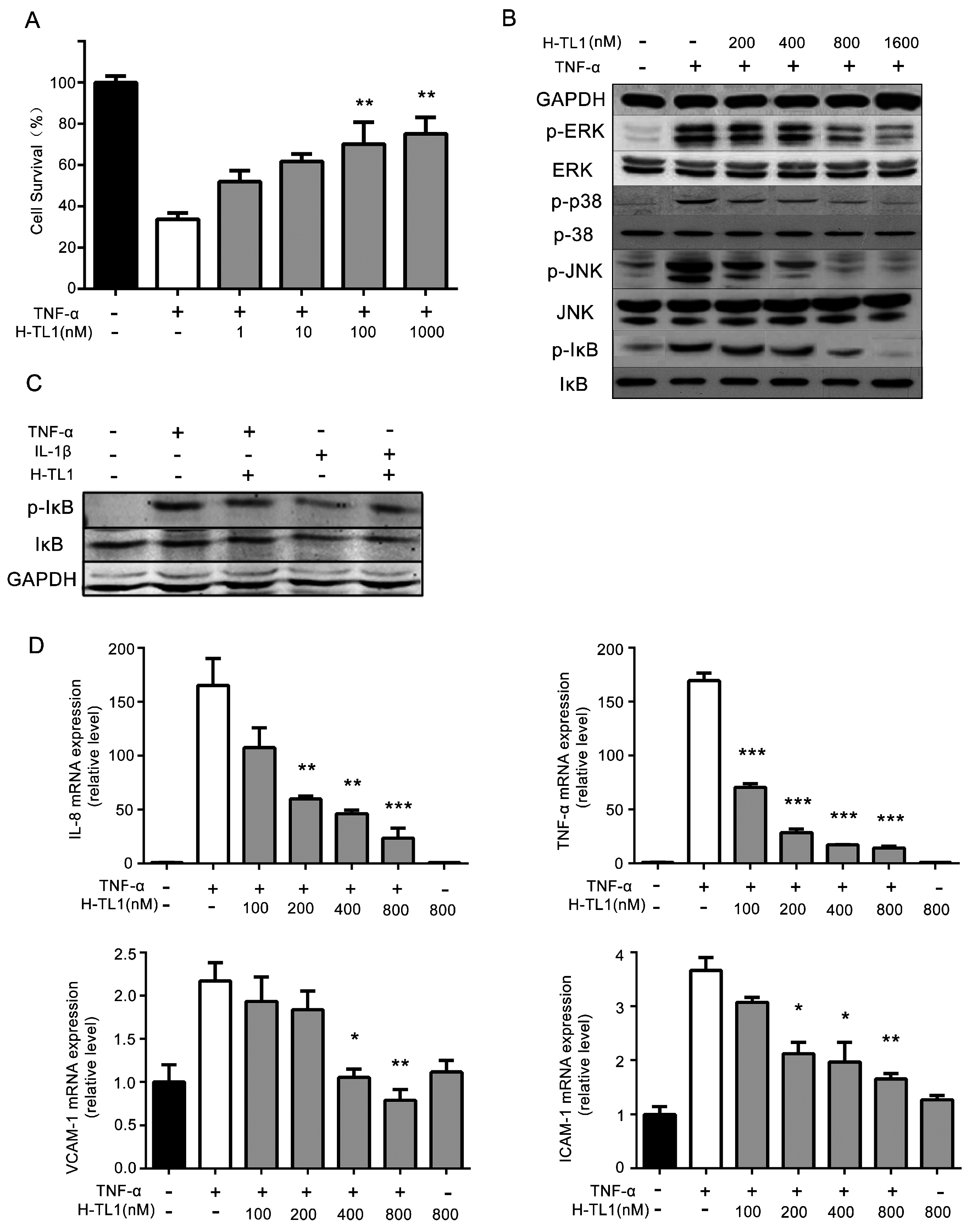
2.3. H-TL1 Reduced the Effects of TNF-α In Vitro
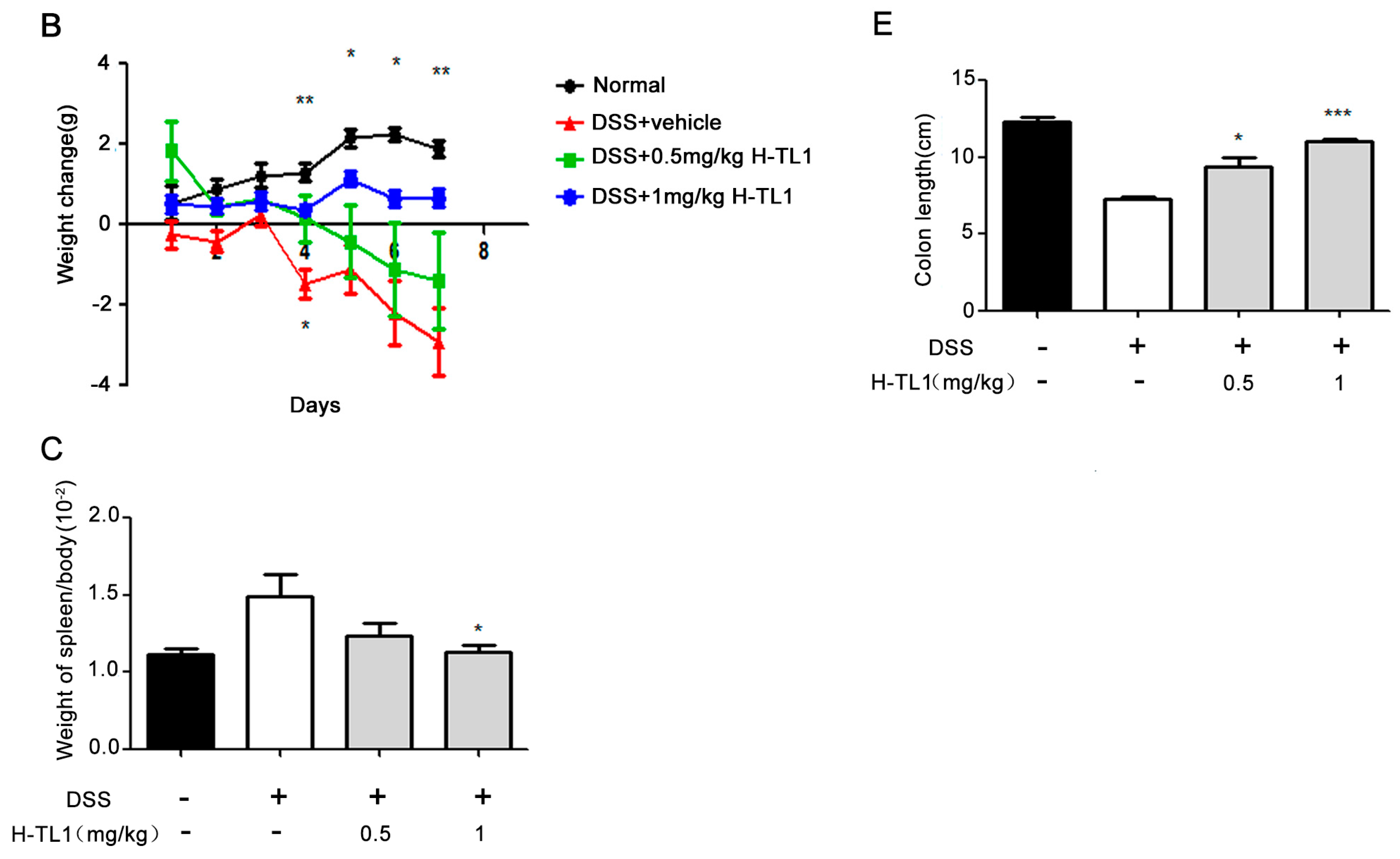
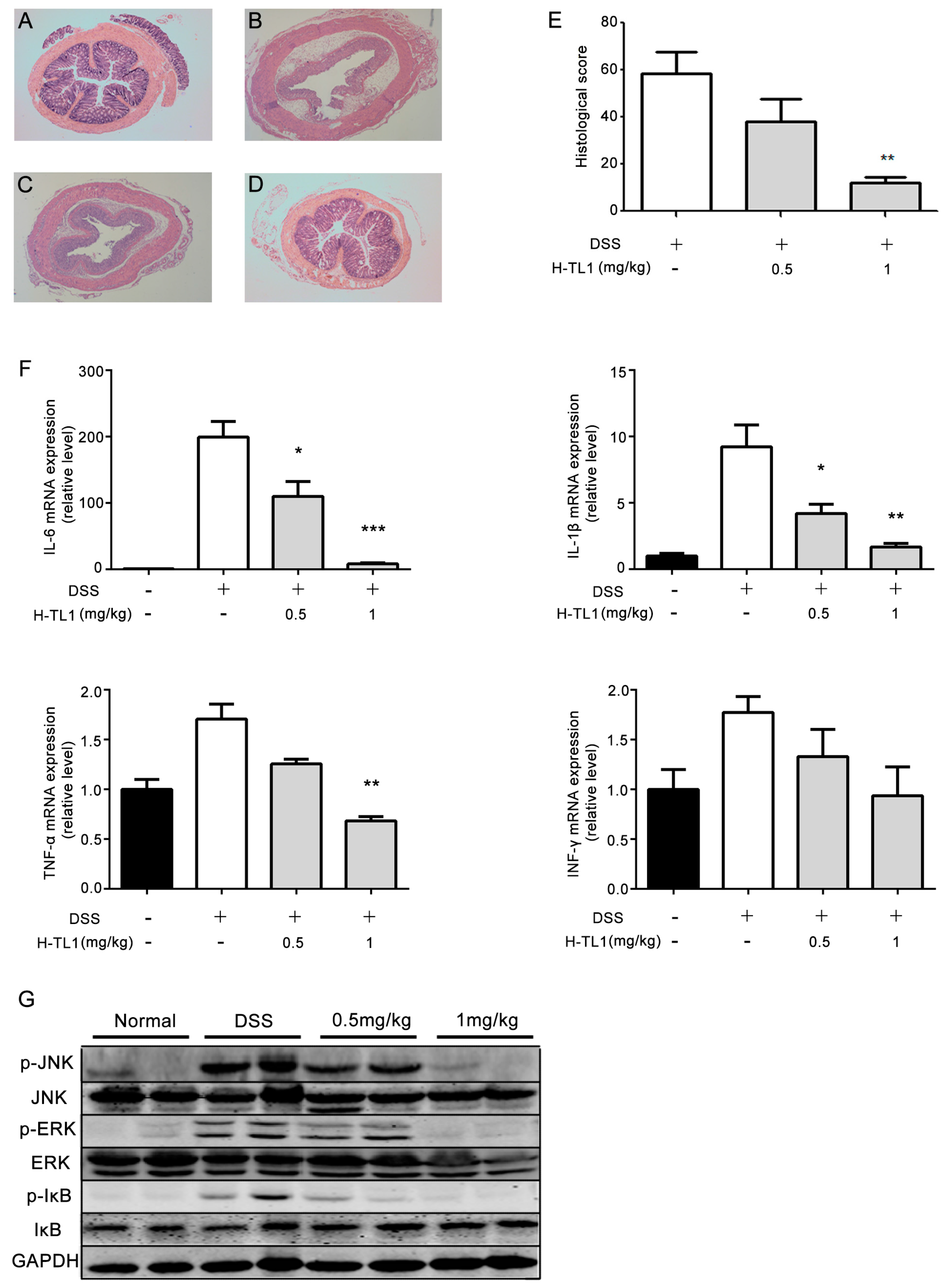
2.4. H-TL1 Alleviated Experimental Colitis in Mice
2.5. Effects of H-TL1 on Histopathology, Cytokines, and Signaling Pathways in Colonic Mucosa
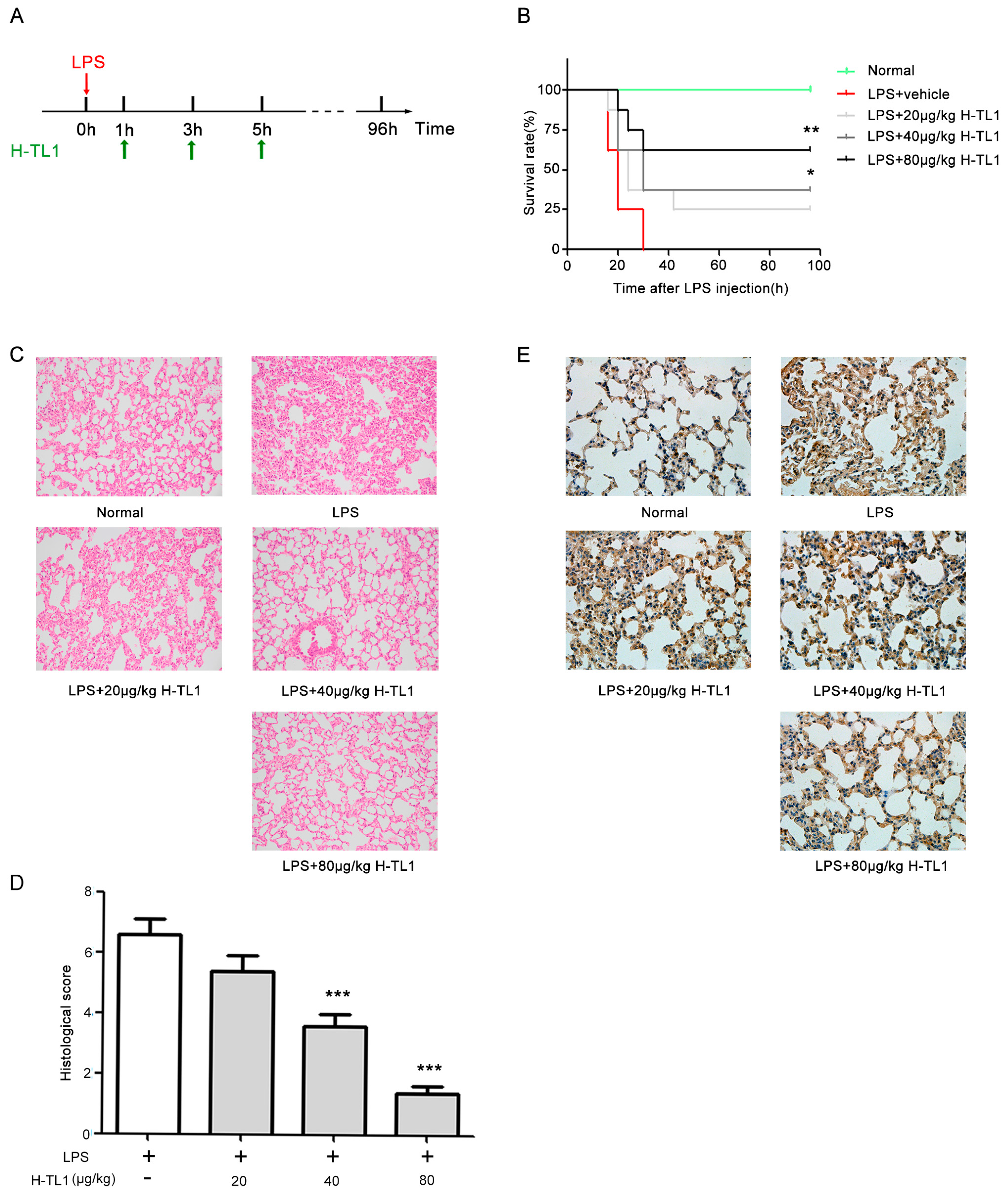
2.6. H-TL1 Reduced Mortality of Mice with LPS-Induced Acute Shock and Pathological Alterations
3. Materials and Methods
3.1. Reagents
3.2. Biological Materials
3.3. RNA Isolation and Construction of T7 Phage Display Library
3.4. Screening and Identification of TNF-α-Binding Peptides Using Phage Display
3.5. Peptide Synthesis
3.6. ELISA Evaluation
3.7. SPR Analysis
3.8. MTT Assay
3.9. Western Blot Analysis
3.10. RNA Extraction and Real-Time PCR
3.11. Induction of Colitis and Treatments
3.12. LPS-Induced Acute Shock and Treatments
3.13. Colon Histopathology
3.14. Lung Histopathology
3.15. Immunohistochemistry
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TNF-α | Tumor necrosis factor-α |
| IBD | Inflammatory bowel disease |
| ELISA | Enzyme-linked immunosorbent assay |
| SPR | Surface plasmon resonance |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DSS | Dextran sodium sulfate |
| LPS | Lipopolysaccharide |
| DAI | Disease activity index |
| IHC | Immunohistochemistry |
References
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Declercq, W.; Beyaert, R.; Fiers, W. Two tumour necrosis factor receptors: Structure and function. Trends Cell Biol. 1995, 5, 392–399. [Google Scholar] [CrossRef]
- Arnett, H.A.; Mason, J.; Marino, M.; Suzuki, K.; Matsushima, G.K.; Ting, J.P. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001, 4, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Zhou, Q.; Howard, O.M.; Netea, M.G.; Oppenheim, J.J. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T Cell phenotype in the inflammatory environment. J. Immunol. 2013, 190, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Grell, M.; Becke, F.M.; Wajant, H.; Mannel, D.N.; Scheurich, P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur. J. Immunol. 1998, 28, 257–263. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm. Bowel Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Colangelo, J. Small-molecule inhibitors of the interaction between TNF and TNFR. Future Med. Chem. 2013, 5, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Laufer, S. Small molecular anti-cytokine agents. Med. Res. Rev. 2006, 26, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-α signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Banner, D.W.; D’Arcy, A.; Janes, W.; Gentz, R.; Schoenfeld, H.J.; Broger, C.; Loetscher, H.; Lesslauer, W. Crystal structure of the soluble human 55 kD TNF receptor-human TNF β complex: Implications for TNF receptor activation. Cell 1993, 73, 431–445. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.; Belasco, J.G. T7 phage display: A novel genetic selection system for cloning RNA-binding proteins from cDNA libraries. Proc. Natl. Acad. Sci. USA 2001, 98, 12954–12959. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Arap, W.; Rajotte, D.; Lahdenranta, J.; Pasqualini, R. Identification of receptor ligands with phage display peptide libraries. J. Nucl. Med. 1999, 40, 883–888. [Google Scholar] [PubMed]
- Sclavons, C.; Burtea, C.; Boutry, S.; Laurent, S.; Vander Elst, L.; Muller, R.N. Phage display screening for tumor necrosis factor-α-binding peptides: Detection of inflammation in a mouse model of hepatitis. Int. J. Pept. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chirinos-Rojas, C.L.; Steward, M.W.; Partidos, C.D. A peptidomimetic antagonist of TNF-α-mediated cytotoxicity identified from a phage-displayed random peptide library. J. Immunol. 1998, 161, 5621–5626. [Google Scholar] [PubMed]
- Tu, A.T. Neurotoxins of animal venoms: Snakes. Annu. Rev. Biochem. 1973, 42, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zong, X.; Zhang, H.; Wang, T.; Yi, H.; Luan, C.; Wang, Y. Antimicrobial peptide Cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int. Immunopharmacol. 2015, 25, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yu, C.; Zhang, H.; Song, D.; Jiang, D.; Du, H.; Wang, Y. Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-κ signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int. Immunopharmacol. 2015, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Nagamizu, M.; Komori, Y.; Uchiya, K.; Nikai, T.; Tu, A.T. Isolation and chemical characterization of a toxin isolated from the venom of the sea snake, Hydrophis torquatus aagardi. Toxins 2009, 1, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and characterization of the first Cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jiang, H.; Huang, Y.; Wang, J.; Qiu, L.; Hu, Z.; Ma, X.; Lu, Y. Screening of an anti-inflammatory peptide from Hydrophis cyanocinctus and analysis of its activities and mechanism in DSS-induced acute colitis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Flick, D.A.; Gifford, G.E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J. Immunol. Methods 1984, 68, 167–175. [Google Scholar] [CrossRef]
- Mishra, S. A spin decay assay for tumor necrosis factor cytotoxicity. Indian J. Biochem. Biophys. 1995, 32, 254–260. [Google Scholar] [PubMed]
- Hu, Z.; Qin, J.; Zhang, H.; Wang, D.; Hua, Y.; Ding, J.; Shan, L.; Jin, H.; Zhang, J.; Zhang, W. Japonicone a antagonizes the activity of TNF-α by directly targeting this cytokine and selectively disrupting its interaction with TNF receptor-1. Biochem. Pharmacol. 2012, 84, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.; Kuwahara, M.; Takada, Y.; Maruyama, K.; Kawaguchi, T.; Tsubone, H.; Ishikawa, H.; Matsuo, K. c-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 2006, 18, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Elisei, W.; Picchio, M.; Giorgetti, G.; Brandimarte, G. Accuracy of rapid fecal calprotectin test in monitoring inflammatory bowel diseases under treatment with TNFα antagonists. Dig. Dis. Sci. 2015, 60, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L.; BonDurant, R.H.; Corbeil, R.R.; Corbeil, L.B. Immune and inflammatory responses to reproductive tract infection with Tritrichomonas foetus in immunized and control heifers. J. Parasitol. 1996, 82, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Mantovani, A. Inflammatory bowel disease and intestinal cancer: A paradigm of the yin-yang interplay between inflammation and cancer. Oncogene 2010, 29, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Raza, A. Anti-TNF therapies in rheumatoid arthritis, Crohn’s disease, sepsis, and myelodysplastic syndromes. Microsc. Res. Tech. 2000, 50, 229–235. [Google Scholar] [CrossRef]
- Simpson, S.Q.; Casey, L.C. Role of tumor necrosis factor in sepsis and acute lung injury. Crit. Care Clin. 1989, 5, 27–47. [Google Scholar] [PubMed]
- Song, Z.; Song, Y.; Yin, J.; Shen, Y.; Yao, C.; Sun, Z.; Jiang, J.; Zhu, D.; Zhang, Y.; Shen, Q.; et al. Genetic variation in the TNF gene is associated with susceptibility to severe sepsis, but not with mortality. PLoS ONE 2012, 7, e46113. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kang, J.H.; Kim, D.K.; Oh, S.H.; Kim, M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, N.; Zhu, C.; Zhou, D.; Nie, T.; Go, M.F.; Richards, R.J.; Rigas, B. MC-12, an annexin A1-based peptide, is effective in the treatment of experimental colitis. PLoS ONE 2012, 7, e41585. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Takuma, S.; Morimoto, M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp. Anim. 2000, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, C.F.; Cerwinka, W.H.; Laroux, F.S.; Grisham, M.B.; Schurmann, G.; Bruwer, M.; Granger, D.N. Role of appendix and spleen in experimental colitis. J. Surg. Res. 2001, 101, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Benavides, U.; Gonzalez-Murguiondo, M.; Harii, N.; Lewis, C.J.; Sakhalkar, H.S.; Deosarkar, S.P.; Kurjiaka, D.T.; Dagia, N.M.; Goetz, D.J.; Kohn, L.D. Phenyl methimazole suppresses dextran sulfate sodium-induced murine colitis. Eur. J. Pharmacol. 2010, 643, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Mabley, J.; Soriano, F.; Pacher, P.; Hasko, G.; Marton, A.; Wallace, R.; Salzman, A.; Szabo, C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur. J. Pharmacol. 2003, 466, 323–329. [Google Scholar] [CrossRef]
- Moreira, V.; Dos-Santos, M.C.; Nascimento, N.G.; Borges da Silva, H.; Fernandes, C.M.; D’Imperio Lima, M.R.; Teixeira, C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon 2012, 60, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Yao, L.; Zhang, B.; Zhang, S.; Guo, J. Anti-inflammatory effects of Neurotoxin-Nna, a peptide separated from the venom of Naja naja atra. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, C.W.; Silva, C.M.; Wong, D.V.; Ximenes, R.M.; Morelo, D.F.; Cosker, F.; Aragao, K.S.; Fernandes, C.; Palheta-Junior, R.C.; Havt, A.; et al. Bothrops jararacussu snake venom-induces a local inflammatory response in a prostanoid- and neutrophil-dependent manner. Toxicon 2014, 90, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Fernandes, C.M.; Zamuner, S.R.; Gutierrez, J.M.; Teixeira, C.F. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: Role of catalytic activity. Toxicon 2005, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]

| Scores | Body Weight Loss (%) | Stool Consistency | Presence or Absence of Fecal Blood |
|---|---|---|---|
| 0 | none | well-formed pellets | none |
| 1 | 1–5 | well-formed pellets | none |
| 2 | 5–10 | loose stools | slight bleeding |
| 3 | 10–20 | loose stools | slight bleeding |
| 4 | >20 | diarrhea | gross bleeding |
| Scores | Severity of Inflammation | Extent of Inflammation | Crypt Damage |
|---|---|---|---|
| 0 | none | none | none |
| 1 | mild | mucosal | basal 1/3 |
| 2 | moderate | mucosal and submucosal | basal 2/3 |
| 3 | severe | transmural | crypts lost but surface epithelium present |
| 4 | - | - | crypts and surface epithelium lost |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Huang, Y.; Li, A.; Jiang, H.; Wang, J.; Li, J.; Qiu, L.; Li, K.; Lu, Y. Hydrostatin-TL1, an Anti-Inflammatory Active Peptide from the Venom Gland of Hydrophis cyanocinctus in the South China Sea. Int. J. Mol. Sci. 2016, 17, 1940. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111940
Wang N, Huang Y, Li A, Jiang H, Wang J, Li J, Qiu L, Li K, Lu Y. Hydrostatin-TL1, an Anti-Inflammatory Active Peptide from the Venom Gland of Hydrophis cyanocinctus in the South China Sea. International Journal of Molecular Sciences. 2016; 17(11):1940. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111940
Chicago/Turabian StyleWang, Ningyuan, Yan Huang, An Li, Hailong Jiang, Jie Wang, Jianzhong Li, Lei Qiu, Ka Li, and Yiming Lu. 2016. "Hydrostatin-TL1, an Anti-Inflammatory Active Peptide from the Venom Gland of Hydrophis cyanocinctus in the South China Sea" International Journal of Molecular Sciences 17, no. 11: 1940. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111940