Intracranial Thrombus Morphology and Composition Undergoes Time-Dependent Changes in Acute Ischemic Stroke: A CT Densitometry Study
Abstract
:1. Introduction
2. Results
2.1. Patient Eligibility and Characteristics
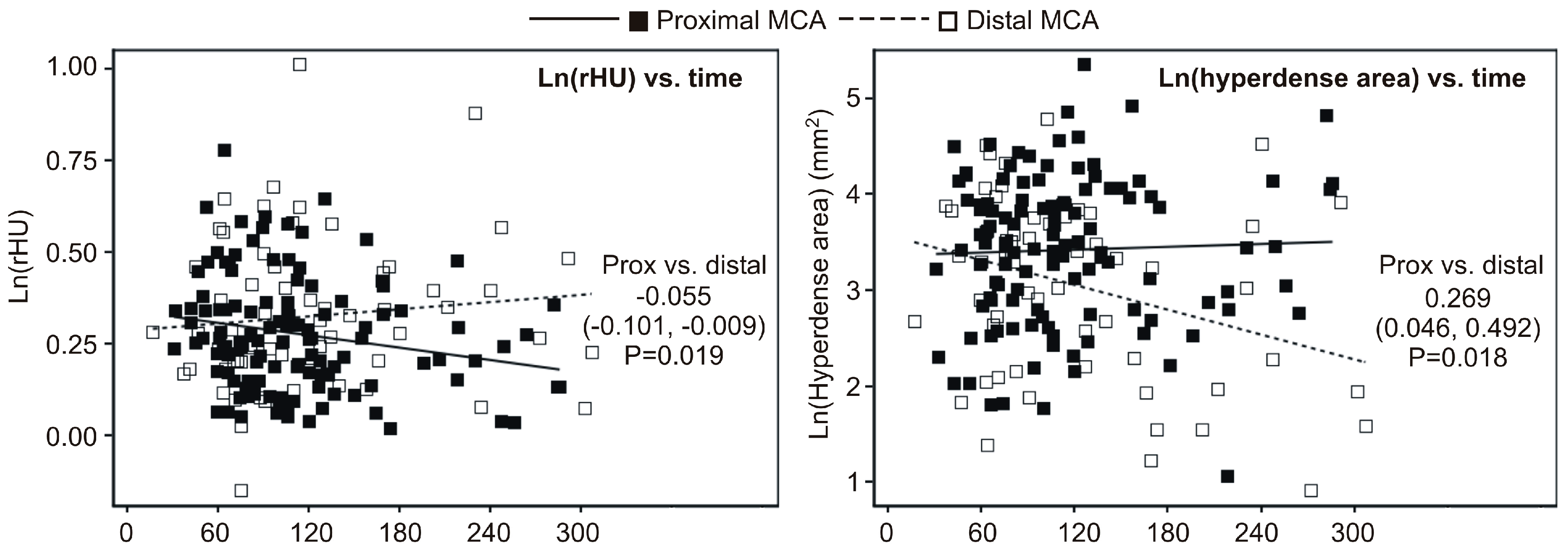
2.2. Univariate Association between Timing of NECT (Non-Contrast-Enhanced CT) Relative to Symptom Onset and Ratio of Density (rHU) or Hyperdense Area
- (a)
- rHU decreased linearly across quartiles of time (linear trend p = 0.025) at the proximal MCA and values at Q4 were 9% lower than at Q1 (p = 0.010) (Figure 1A); at the distal MCA, an apparent cubic trend (p = 0.016) was observed since rHU values declined from Q1 to Q2 and then increased at Q3 and Q4, and hence values at Q4 were actually no different than the values at Q1 (Figure 3A);
- (b)
- At the proximal MCA, there was no apparent difference regarding the hyperdense area across quartiles of time (Figure 3B), whereas at distal MCA there was a significant cubic trend (p = 0.017)—the values slightly increased from Q1 to Q3, and then declined at Q4, so that the values at Q4 were 42% lower than at Q1 (p = 0.020) (Figure 3B).
2.3. Multivariate (Independent) Association between Timing of NECT Relative to Symptom Onset and Ratio of Density (rHU) or Hyperdense Area
- (a)
- At the proximal MCA, the rHU linearly decreased across quartiles of the time-lag (linear trend p = 0.019) and values at the fourth quartile were 10% lower than at the first quartile (p = 0.008); at the distal MCA, the cubic trend remained significant and there was no difference in rHU at the fourth vs. first quartile of the time-lag; and
- (b)
- At the proximal MCA there was no apparent change in the hyperdense area across quartiles of the time-lag, whereas at the distal MCA the cubic trend remained significant and values at the fourth quartile were 39% lower than at the first quartile (Table 2).
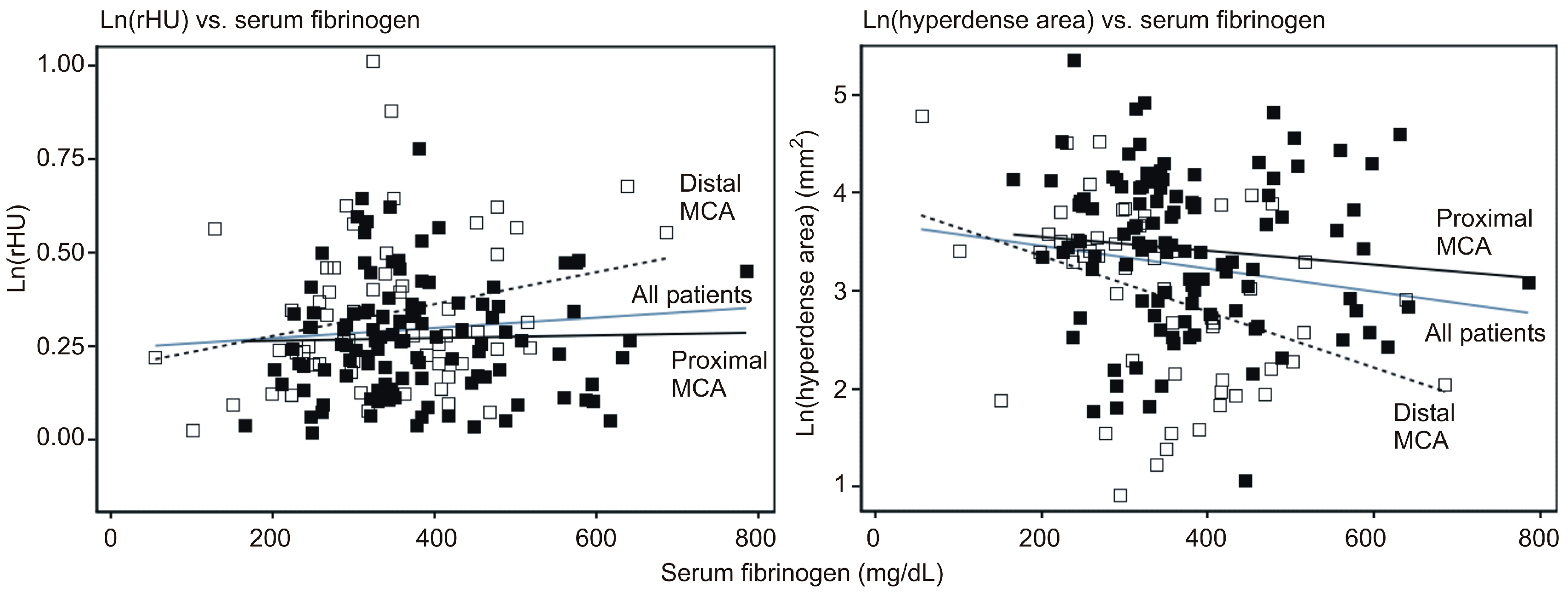
2.4. Exploration of the Relationship between Serum Fibrinogen Levels and rHU or Hyperdense Area
- (a)
- there was an overall trend of association between higher serum fibrinogen and higher rHU (2.3% higher with 100 mg/dL increase in fibrinogen). However, there was no association between fibrinogen and rHU in patients with an affected proximal MCA, whereas the association was stronger and statistically significant in patients with an affected distal MCA (4.2% higher rHU by 100 mg/dL increase in fibrinogen);
- (b)
- for the entire cohort, higher fibrinogen was associated with a smaller hyperdense area (15% by 100 mg/dL increase in fibrinogen) (p = 0.005). However, this association was much weaker and not statistically significant in patients with an affected proximal MCA, whereas it was stronger and significant in patients with an affected distal MCA (Table 3). Due to incompleteness, data should be viewed with caution, but suggest that at proximal MCA, rHU apparently declines over the first 300 min after the stroke onset, though the hyperdense area does not appear to change. Further, neither of these two radiological outcomes seems to be associated with serum fibrinogen levels. Moreover, at the distal MCA, rHU does not appear to change while the hyperdense area tends to diminish over the first 300 min after the stroke onset. At the same time, a higher rHU and lower hyperdense area appear to be associated with higher serum fibrinogen.
3. Discussion
4. Materials and Methods
Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| LAA | Large artery atherosclerosis |
| HAS | Hyperdense artery sign |
| NECT | Non-enhanced CT |
| TOAST | Trial of Org 10172 in Acute Treatment criteria |
| HU | Hounsfield units |
| rHU | Average HU of hyperdense artery/average HU of non-affected artery |
| DSA | Digital subtraction angiography |
| MRI | Magnetic resonance imaging |
| CT | Computertomography |
| ESUS | Embolic stroke of unknown source |
| CE | Cardioembolic |
References
- Williams, G.R.; Jiang, J.G.; Matchar, D.B.; Samsa, G.P. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke 1999, 30, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Trkulja, V.; Malojcic, B.; Mutzenbach, J.S.; Sellner, J. A High Burden of Ischemic Stroke in Regions of Eastern/Central Europe is Largely Due to Modifiable Risk Factors. Curr. Neurovasc. Res. 2015, 12, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Goldmakher, G.V.; Camargo, E.C.; Furie, K.L.; Singhal, A.B.; Roccatagliata, L.; Halpern, E.F.; Chou, M.J.; Biagini, T.; Smith, W.S.; Harris, G.J.; et al. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke 2009, 40, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Marder, V.J.; Chute, D.J.; Starkman, S.; Abolian, A.M.; Kidwell, C.; Liebeskind, D.; Ovbiagele, B.; Vinuela, F.; Duckwiler, G.; Jahan, R.; et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006, 37, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Mattle, H.P.; Arnold, M.; Georgiadis, D.; Baumann, C.; Nedeltchev, K.; Benninger, D.; Remonda, L.; von Budingen, C.; Diana, A.; Pangalu, A.; et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke 2008, 39, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; Biessels, G.J.; van Otterloo, A.E.; van Seeters, T.; Horsch, A.D.; Luitse, M.J.; van der Graaf, Y.; Kappelle, L.J.; Mali, W.P.; et al. Relationship between thrombus attenuation and different stroke subtypes. Neuroradiology 2013, 55, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Gold, H.K.; Ziskind, A.A.; Fallon, J.T.; Holt, R.E.; Leinbach, R.C.; May, J.W.; Collen, D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation 1989, 79, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S.; Sanossian, N.; Yong, W.H.; Starkman, S.; Tsang, M.P.; Moya, A.L.; Zheng, D.D.; Abolian, A.M.; Kim, D.; Ali, L.K.; et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011, 42, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Trkulja, V.; Mutzenbach, J.S.; McCoy, M.R.; Ganger, P.; Sellner, J. Fibrinogen consumption is related to intracranial clot burden in acute ischemic stroke: A retrospective hyperdense artery study. J. Transl. Med. 2016, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Mair, G.; Boyd, E.V.; Chappell, F.M.; von Kummer, R.; Lindley, R.I.; Sandercock, P.; Wardlaw, J.M.; Group, I.S.T.C. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke 2015, 46, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Pedraza, S.; Demchuk, A.; Daunis, I.E.J.; Termes, H.; Blasco, G.; Soria, G.; Boada, I.; Remollo, S.; Banos, J.; et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. Am. J. Neuroradiol. 2012, 33, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.H.; Jensen, U.; Rohr, A.; Tietke, M.; Alfke, K.; Ulmer, S.; Jansen, O. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced Computed Tomography reconstructions. Stroke 2010, 41, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; van der Graaf, Y.; Kappelle, L.J.; Biessels, G.J.; Horsch, A.D.; Dankbaar, J.W.; Luitse, M.J.; van Seeters, T.; Smit, E.J.; et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc. Dis. 2014, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Morr, S.; Natarajan, S.K.; Lin, N.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J. Neurointerv. Surg. 2015, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, L.; Lovblad, K.O.; Kulcsar, Z. Pretherapeutic characterization of the clot in acute stroke. J. Neuroradiol. 2016, 43, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.H.; Zimmermann, P.; Jensen-Kondering, U.; Stingele, R.; Deuschl, G.; Jansen, O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011, 42, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A. Imaging the clot: Does clot appearance predict the efficacy of thrombolysis? Stroke 2005, 36, 2333–2334. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, N.; Lucero, J.; Heo, J.H.; Koziol, J.A.; Copeland, B.R.; del Zoppo, G.J. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke 2001, 32, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, F.J.; Castelo-Branco, M.; Alvarez-Sabin, J. Usefulness of measurement of fibrinogen, D-dimer, D-dimer/fibrinogen ratio, C reactive protein and erythrocyte sedimentation rate to assess the pathophysiology and mechanism of ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2011, 82, 986–992. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Denorme, F.; Langhauser, F.; Geuss, E.; Fluri, F.; Kleinschnitz, C. Thromboinflammation in Stroke Brain Damage. Stroke 2016, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, M.A.; Arsava, E.M.; Akpinar, E. Clot Characteristics on Computed Tomography and Response to Thrombolysis in Acute Middle Cerebral Artery Stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Haridy, J.; Churilov, L.; Mitchell, P.; Dowling, R.; Yan, B. Is there association between hyperdense middle cerebral artery sign on CT scan and time from stroke onset within the first 24-hours? BMC Neurol. 2015, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.C.; Connolly, S.J. Embolic strokes of undetermined source: Support for a new clinical construct—Authors’ reply. Lancet Neurol. 2014, 13, 967. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Ferro, J.M. Cryptogenic stroke. Eur. J. Neurol. 2015, 22, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Scullen, T.A.; Monlezun, D.J.; Siegler, J.E.; George, A.J.; Schwickrath, M.; El Khoury, R.; Cho, M.C.; Martin-Schild, S. Cryptogenic stroke: Clinical consideration of a heterogeneous ischemic subtype. J. Stroke Cerebrovasc. Dis. 2015, 24, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Krings, T.; Noelchen, D.; Mull, M.; Willmes, K.; Meister, I.G.; Reinacher, P.; Toepper, R.; Thron, A.K. The hyperdense posterior cerebral artery sign: A computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke 2006, 37, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Kolominsky-Rabas, P.L.; Weber, M.; Gefeller, O.; Neundoerfer, B.; Heuschmann, P.U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke 2001, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.C.; Morris, T.P. Adjusting for multiple prognostic factors in the analysis of randomised trials. BMC Med. Res. Methodol. 2013, 13, 99. [Google Scholar] [CrossRef] [PubMed]


| Variables | All Patients | Proximal MCA | Distal MCA | |||
|---|---|---|---|---|---|---|
| N | Values | N | Values | N | Values | |
| Age (years) | 185 | 75 (19–98) | 118 | 75 (41–97) | 67 | 75 (19–98) |
| Men | 185 | 82 (44.3) | 118 | 52 (44.1) | 67 | 30 (44.8) |
| Symptom onset to NECT (min) | 185 | 100 (17–300) (Q1–Q3 = 71–136) | 118 | 104 (31–286) (Q1–Q3 = 71–133) | 67 | 94 (17–300) (Q1–Q3 = 70–147) |
| Side affected (left/right) | 185 | 92 (49.7)/93 | 118 | 62 (52.5)/56 | 67 | 30 (44.8)/37 |
| Average clot density (HU) | 185 | 46.3 (36.1–56.1) | 118 | 46.5 (36.9–56.1) | 67 | 45.9 (36.1–55.3) |
| Non-affected side density (HU) | 185 | 35.8 (18.6–45.7) | 118 | 35.7 (24.4–45.7) | 67 | 33.9 (18.6–45.7) |
| Ratio clot/non-affected rHU | 185 | 1.30 (0.86–2.75) | 118 | 1.30 (1.02–2.18) | 67 | 1.32 (0.86–2.75) |
| Hyperdense area (mm2) | 176 | 30.2 (2.5–211.4) | 115 | 31.7 (2.9–211.4) | 61 | 28.2 (2.5–119.0) |
| Admission NIHSS | 185 | 16 (0–32) | 118 | 17 (0–32) | 67 | 13 (0–32) |
| TOAST class | 185 | 118 | 67 | |||
| Cardioembolic | 93 (50.3) | 59 (50.0) | 34 (50.8) | |||
| Large artery atherosclerosis | 23 (12.4) | 20 (16.9) | 3 (4.5) | |||
| Other (all arterial dissections) | 6 (3.2) | 4 (3.4) | 2 (3.0) | |||
| Undetermined | 9 (4.9) | 3 (2.5) | 6 (8.9) | |||
| Unknown | 54 (29.1) | 32 (27.1) | 22 (32.8) | |||
| Angiography performed * | 185 | 118 (63.8) | 118 | 89 (75.4) | 67 | 29 (43.3) |
| Thrombolysis | 185 | 139 (75.1) | 118 | 90 (76.3) | 67 | 49 (73.1) |
| Thrombectomy | 185 | 52 (28.1) | 118 | 48 (40.7) | 67 | 4 (6.0) |
| Thrombolysis + thrombectomy | 185 | 44 (23.8) | 118 | 40 (33.9) | 67 | 4 (6.0) |
| Usage of antiplatelets | 185 | 55 (29.7) | 118 | 33 (28.0) | 67 | 22 (32.8) |
| Usage of anticoagulants | 185 | 19 (10.3) | 118 | 12 (10.2) | 67 | 7 (10.5) |
| History of stroke or TIA | 185 | 25 (13.5) | 118 | 14 (11.9) | 67 | 11 (16.4) |
| Peripheral arterial disease | 185 | 14 (7.6) | 118 | 9 (7.6) | 67 | 5 (7.5) |
| Atrial fibrillation | 185 | 91 (49.5) | 118 | 56 (47.5) | 67 | 35 (53.0) |
| Diabetes mellitus | 185 | 31 (16.8) | 118 | 19 (16.1) | 67 | 12 (17.9) |
| Arterial hypertension | 185 | 127 (68.6) | 118 | 84 (71.2) | 67 | 43 (64.2) |
| Carotid stenosis >50% | 185 | 24 (13.0) | 118 | 16 (13.6) | 67 | 8 (11.9) |
| Chronic heart failure | 185 | 30 (16.2) | 118 | 21 (17.8) | 67 | 9 (13.4) |
| Blood glucose (mg/dL) | 184 | 119 (76–351) | 118 | 119 (76–254) | 66 | 120 (77–351) |
| Total cholesterol (mg/dL) | 160 | 181 (78–300) | 102 | 185 (78–300) | 58 | 175 (99–275) |
| Serum fibrinogen (mg/dL) | 170 | 346 (55–785) | 111 | 350 (166–785) | 59 | 335 (55–685) |
| Associations | At Proximal MCA | At Distal MCA | ||
|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Dependent: rHU | ||||
| Linear trend across time-lag quartiles | −0.280 (−0.513, −0.046) | 0.019 | 0.230 (−0.224, 0.684) | 0.315 |
| Cubic trend across time-lag quartiles | −0.169 (−0.404, 0.066) | 0.158 | −0.480 (−0.914, −0.046) | 0.031 |
| Difference in fourth to first quartile (%) | −10 (−16, −3) | 0.008 | 2 (−11, 17) | 0.761 |
| Dependent: hyperdense area | ||||
| Linear trend across time-lag quartiles | 0.652 (−0.543, 1.848) | 0.282 | −0.976 (−2.586, 0.671) | 0.243 |
| Cubic trend across time-lag quartiles | −0.906 (−2.104, 0.292) | 0.137 | −2.092 (−3.672, −0.512) | 0.011 |
| Difference in fourth to first quartile (%) | 11 (−24, 62) | 0.581 | −39 (−63, −1) | 0.046 |
| Affected Artery | rHU | Hyperdense Area (mm2) | ||
|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Proximal and distal MCA | 2.27% (−0.10, 4.67) | 0.059 | −15.2% (−24.3, −5.0) | 0.005 |
| Proximal MCA | 0.41% (−2.23, 3.17) | 0.769 | −8.2% (−29.3, 4.5) | 0.191 |
| Distal MCA | 4.16% (0.39, 8.08) | 0.031 | −21.7% (−34.8, −5.8) | 0.009 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikija, S.; Magdic, J.; Trkulja, V.; Unterkreuter, P.; Mutzenbach, J.S.; Novak, H.F.; Weymayr, F.; Hauer, L.; Sellner, J. Intracranial Thrombus Morphology and Composition Undergoes Time-Dependent Changes in Acute Ischemic Stroke: A CT Densitometry Study. Int. J. Mol. Sci. 2016, 17, 1959. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111959
Pikija S, Magdic J, Trkulja V, Unterkreuter P, Mutzenbach JS, Novak HF, Weymayr F, Hauer L, Sellner J. Intracranial Thrombus Morphology and Composition Undergoes Time-Dependent Changes in Acute Ischemic Stroke: A CT Densitometry Study. International Journal of Molecular Sciences. 2016; 17(11):1959. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111959
Chicago/Turabian StylePikija, Slaven, Jozef Magdic, Vladimir Trkulja, Peter Unterkreuter, Johannes Sebastian Mutzenbach, Helmut F. Novak, Friedrich Weymayr, Larissa Hauer, and Johann Sellner. 2016. "Intracranial Thrombus Morphology and Composition Undergoes Time-Dependent Changes in Acute Ischemic Stroke: A CT Densitometry Study" International Journal of Molecular Sciences 17, no. 11: 1959. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111959





