Effects of Atorvastatin Dose and Concomitant Use of Angiotensin-Converting Enzyme Inhibitors on Renal Function Changes over Time in Patients with Stable Coronary Artery Disease: A Prospective Observational Study
Abstract
:1. Introduction
2. Results
| Characteristic | Atorvastatin Daily Dose | p-Value | ||
|---|---|---|---|---|
| 10-mg, n = 29 | 20-mg, n = 24 | 40-mg, n = 25 | ||
| Age (years) | 56 ± 10 | 57 ± 9 | 55 ± 10 | NS |
| Hypertension, n (%) | 25 (86%) | 19 (79%) | 20 (80%) | NS |
| Diabetes, n (%) | 9 (31%) | 6 (25%) | 5 (20%) | NS |
| Left ventricular ejection fraction (%) | 68 ± 6 | 70 ± 7 | 70 ± 6 | NS |
| Body-mass index (kg/m2) | 27.4 ± 3.4 | 26.8 ± 2.9 | 27.0 ± 3.1 | NS |
| Mean blood pressure (mmHg) | 99 ± 9 | 97 ± 9 | 96 ± 8 | NS |
| LDL cholesterol (mmol/L) | 2.1 ± 0.3 | 2.0 ± 0.4 | 2.2 ± 0.3 | NS |
| HDL cholesterol (mmol/L) | 0.9 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | NS |
| Triglycerides (mmol/L) | 1.7 ± 0.7 | 1.6 ± 0.6 | 1.5 ± 0.6 | NS |

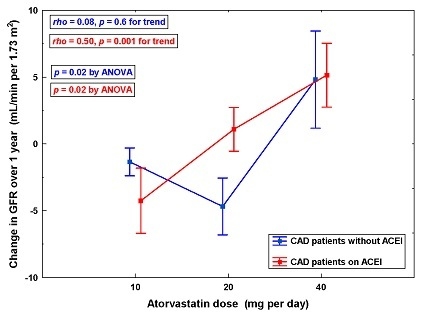
| GFR (mL/min per 1.73 m2) | Atorvastatin Daily Dose | p-Value by ANOVA | p-Value for Trend | ||
|---|---|---|---|---|---|
| 10-mg | 20-mg | 40-mg | |||
| Patients on ACEI (n = 38) | |||||
| n = 13 | n = 11 | n = 14 | |||
| Baseline | 65.9 ± 4.8 | 74.7 ± 6.2 | 69.0 ± 4.0 | NS | NS |
| Change over 1 year | −4.2 ± 2.4 | 1.1 ± 1.6 | 5.2 ± 2.4 * | 0.02 | 0.001 |
| Patients without ACEI (n = 40) | |||||
| n = 16 | n = 13 | n = 11 | |||
| Baseline | 41.4 ± 4.5 | 49.3 ± 5.3 | 41.3 ± 4.3 | NS | NS |
| Change over 1 year | −1.3 ± 1.0 | −4.7 ± 2.1 | 4.8 ± 3.6 † | 0.02 | NS |

| Predictor | Regression Coefficient | p-Value | |
|---|---|---|---|
| Mean ± SEM | 95% CI | ||
| Atorvastatin daily dose | |||
| 40-mg vs. 10-mg | 3.9 ± 1.1 | 1.8–6.0 | <0.001 |
| 20-mg vs. 10-mg | 0.5 ± 1.1 | −1.7–2.7 | 0.7 |
| Treatment with ACEI (yes vs. no) | 1.5 ± 1.2 | −0.7–3.8 | 0.2 |
| Interactions of ACEI use and atorvastatin dose | |||
| ACEI use and 20-mg atorvastatin | 2.3 ± 1.1 | 0.1–4.5 | 0.04 |
| ACEI use and 40-mg atorvastatin | 0.8 ± 1.1 | −1.3–2.9 | 0.5 |
3. Discussion
3.1. Mechanistic Considerations
3.2. Clinical Implications
3.3. Study Limitations
4. Experimental Section
4.1. Patients
4.2. Collection of Follow-up Data
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitors |
| ANOVA | analysis of variance |
| ARB | angiotensin II type 1 receptor blockers |
| CAD | coronary artery disease |
| CKD | chronic kidney disease |
| CV | cardiovascular |
| GFR | glomerular filtration rate |
| HDL | high-density lipoproteins |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-coenzyme A |
| LDL | low-density lipoproteins |
| NO | nitric oxide |
| NS | non-significant |
| SEM | standard error of the mean |
| SD | standard deviation |
| TGF | tubuloglomerular feedback |
References
- Fried, L.F. Effects of HMG-CoA reductase inhibitors (statins) on progression of kidney disease. Kidney Int. 2008, 74, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Wiebe, N.; Fried, L.F.; Tonelli, M. Statins for improving renal outcomes: A meta-analysis. J. Am. Soc. Nephrol. 2006, 17, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Craig, J.C.; Navaneethan, S.D.; Tonelli, M.; Pellegrini, F.; Strippoli, G.F. Benefits and harms of statin therapy for persons with chronic kidney disease: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Lv, J.; Perkovic, V.; Yang, L.; Zhao, N.; Jardine, M.J.; Cass, A.; Zhang, H.; Wang, H. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: A systematic review and meta-analysis. Eur. Heart J. 2013, 34, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Banach, M.; Nikfar, S.; Salari, P.; Mikhailidis, D.P.; Toth, P.P.; Abdollahi, M.; Ray, K.K.; Pencina, M.J.; Malyszko, J.; et al. A meta-analysis of the role of statins on renal outcomes in patients with chronic kidney disease. Is the duration of therapy important? Int. J. Cardiol. 2013, 168, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Umemoto, T. A meta-analysis of randomized trials for effects of atorvastatin on renal function in chronic kidney disease. Int. J. Cardiol. 2011, 152, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Sanguankeo, A.; Upala, S.; Cheungpasitporn, W.; Ungprasert, P.; Knight, E.L. Effects of statins on renal outcome in chronic kidney disease patients: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0132970. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Ren, J.; Song, J.; Li, S.; Chen, H. Meta-analysis of the effect of statins on renal function. Am. J. Cardiol. 2014, 114, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Garcia, M.A.; Ardissino, D.; Buszman, P.; Camici, P.G.; Crea, F.; Daly, C.; De Backer, G.; Hjemdahl, P.; Lopez-Sendon, J.; et al. The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Guidelines on the management of stable angina pectoris: Executive summary. Eur. Heart J. 2006, 27, 1341–1381. [Google Scholar] [PubMed]
- Fraker, T.D.; Fihn, S.D.; Gibbons, R.J.; Abrams, J.; Chatterjee, K.; Daley, J.; Deedwania, P.C.; Douglas, J.S.; Ferguson, T.B.; Gardin, J.M.; et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. J. Am. Coll. Cardiol. 2007, 50, 2264–2274. [Google Scholar] [PubMed]
- Shepherd, J.; Kastelein, J.J.; Bittner, V.; Deedwania, P.; Breazna, A.; Dobson, S.; Wilson, D.J.; Zuckerman, A.; Wenger, N.K.; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: The Treating to New Targets (TNT) study. Clin. J. Am. Soc. Nephrol. 2007, 2, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.K.; Prais, H.R.; Charlton-Menys, V.; Gittins, M.; Roberts, C.; Davies, R.R.; Moorhouse, A.; Jinadev, P.; France, M.; Wiles, P.G.; et al. Protection against nephropathy in diabetes with atorvastatin (PANDA): A randomized double-blind placebo-controlled trial of high- vs. low-dose atorvastatin. Diabet. Med. 2011, 28, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Campese, V.M. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on renal function. Am. J. Kidney Dis. 2005, 45, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Millar, P.J.; Floras, J.S. Statins and the autonomic nervous system. Clin. Sci. (Lond.) 2014, 126, 401–415. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. The Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar] [PubMed]
- Brouhard, B.H.; Takamori, H.; Satoh, S.; Inman, S.; Cressman, M.; Irwin, K.; Berkley, V.; Stowe, N. The combination of lovastatin and enalapril in a model of progressive renal disease. Pediatr. Nephrol. 1994, 8, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jin, S.Y.; Han, D.C.; Hwang, S.D.; Lee, H.B. Effects of delayed treatment with enalapril and/or lovastatin on the progression of glomerulosclerosis in 5/6 nephrectomized rats. Nephrol. Dial. Transplant. 1993, 8, 1338–1343. [Google Scholar] [PubMed]
- Zoja, C.; Corna, D.; Rottoli, D.; Cattaneo, D.; Zanchi, C.; Tomasoni, S.; Abbate, M.; Remuzzi, G. Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kidney Int. 2002, 61, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Corna, D.; Gagliardini, E.; Conti, S.; Arnaboldi, L.; Benigni, A.; Remuzzi, G. Adding a statin to a combination of ACE inhibitor and ARB normalizes proteinuria in experimental diabetes, which translates into full renoprotection. Am. J. Physiol. Ren. Physiol. 2010, 299, F1203–F1211. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Bäumer, A.T.; Temur, Y.; Kebben, D.; Jockenhövel, F.; Böhm, M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation 1999, 100, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Takeda, K.; Tokunou, T.; Iino, N.; Egashira, K.; Shimokawa, H.; Hirano, K.; Kanaide, H.; Takeshita, A. Downregulation of angiotensin II type 1 receptor by hydrophobic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Launay-Vacher, V.; Izzedine, H.; Deray, G. Statins' dosage in patients with renal failure and cyclosporine drug-drug interactions in transplant recipient patients. Int. J. Cardiol. 2005, 101, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Mikhailidis, D.P.; Papageorgiou, A.A.; Symeonidis, A.N.; Pehlivanidis, A.N.; Bouloukos, V.I.; Elisaf, M. The effect of statins vs. untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J. Clin. Pathol. 2004, 57, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Stowe, N.T.; Inman, S.R.; Tapolyai, M.; Brouhard, B.H.; Hodge, E.E.; Novick, A.C. Lovastatin has direct renal hemodynamic effects in a rodent model. J. Urol. 1996, 156, 249–252. [Google Scholar] [CrossRef]
- Ott, C.; Schlaich, M.P.; Schmidt, B.M.; Titze, S.I.; Schäufele, T.; Schmieder, R.E. Rosuvastatin improves basal nitric oxide activity of the renal vasculature in patients with hypercholesterolemia. Atherosclerosis 2008, 196, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Mose, F.H.; Larsen, T.; Bech, J.N.; Pedersen, E.B. Effects of atorvastatin on systemic and renal nitric oxide in healthy man. Clin. Exp. Hypertens. 2013, 35, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.A.; Kamper, A.M.; van Veen, S.; Souverijn, J.H.; Blauw, G.J. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2001, 16, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, F.V.; van de Ree, M.A.; Bots, M.L.; Stolk, R.P.; Huisman, M.V.; Banga, J.D.; DALI Study Group. Aggressive lipid lowering does not improve endothelial function in type 2 diabetes: The Diabetes Atorvastatin Lipid Intervention (DALI) Study: A randomized, double-blind, placebo-controlled trial. Diabetes Care 2002, 25, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Pham, V.V.; Suchy, M.T.; van de Ree, M.A.; Huisman, M.V.; Frölich, J.C.; Princen, H.M.; DALI Study Group. No effects of atorvastatin (10 mg/d or 80 mg/d) on prostacyclin, thromboxane and oxidative stress in type 2 diabetes mellitus patients of the DALI study. Pharmacol. Res. 2015, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Braam, B.; Koomans, H.A. Nitric oxide antagonizes the actions of angiotensin II to enhance tubuloglomerular feedback responsiveness. Kidney Int. 1995, 48, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lu, Y.; Lai, E.Y.; Wei, J.; Wang, L.; Chandrashekar, K.; Wang, S.; Shen, C.; Juncos, L.A.; Liu, R. Oxidative status in the macula densa modulates tubuloglomerular feedback responsiveness in angiotensin II-induced hypertension. Acta. Physiol. (Oxf.) 2015, 213, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Mervaala, E.M.; Muller, D.N.; Menne, J.; Fiebeler, A.; Luft, F.C.; Haller, H. Rosuvastatin protects against angiotensin II-induced renal injury in a dose-dependent fashion. J. Hypertens. 2009, 27, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.S.; Schuman, I.H.; Jaimes, E.A.; Raij, L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am. J. Physiol. Ren. Physiol. 2008, 295, F53–F59. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, F.A.; Rathore, S.S.; Wang, Y.; Havranek, E.P.; Curtis, J.P.; Foody, J.M.; Krumholz, H.M. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation 2004, 110, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Davidson, M.H.; Wilson, D.J.; Fayyad, R.S.; Zuckerman, A.; Reed, D.P.; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am. J. Kidney Dis. 2009, 53, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Mikhailidis, D.P.; Papageorgiou, A.A.; Bouloukos, V.I.; Pehlivanidis, A.N.; Symeonidis, A.N.; Elisaf, M.; Group, G.S.C. Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease. J. Hum. Hypertens. 2004, 18, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Clerici, G.; Scatena, A.; Caminiti, M.; Curci, V.; Morabito, A.; Prisco, V.; Greco, R.; Edmonds, M. Effectiveness of combined therapy with angiotensin-converting enzyme inhibitors and statins in reducing mortality in diabetic patients with critical limb ischemia: An observational study. Diabetes Res. Clin. Pract. 2014, 103, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Bigazzi, R.; Caiazza, A.; Campese, V.M. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am. J. Kidney Dis. 2003, 41, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Robertson, I.K.; Ball, M.J.; Geraghty, D.P.; Coombes, J.S. Effect of atorvastatin on kidney function in chronic kidney disease: A randomised double-blind placebo-controlled trial. Atherosclerosis 2010, 213, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Surdacki, A.; Marewicz, E.; Wieteska, E.; Szastak, G.; Rakowski, T.; Wieczorek-Surdacka, E.; Dudek, D.; Pryjma, J.; Dubiel, J.S. Association between endothelial progenitor cell depletion in blood and mild-to-moderate renal insufficiency in stable angina. Nephrol. Dial. Transplant. 2008, 23, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Surdacki, A.; Marewicz, E.; Wieczorek-Surdacka, E.; Rakowski, T.; Szastak, G.; Pryjma, J.; Dudek, D.; Dubiel, J.S. Synergistic effects of asymmetrical dimethyl-l-arginine accumulation and endothelial progenitor cell deficiency on renal function decline during a 2-year follow-up in stable angina. Nephrol. Dial. Transplant. 2010, 25, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.; Sirois, M.J. Spearman Correlation Coefficients, Differences between. In Encyclopedia of Statistical Science; Kotz, S., Read, C.B., Balakrishnan, N., Vidakovic, B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; Available online: http://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/10.1002/0471667196.ess5050.pub2/abstract (accessed on 20 November 2015). [CrossRef]
- Yanez, N.D.; Kronmal, R.A.; Shemanski, L.R.; Psaty, B.M.; Study, C.H. A regression model for longitudinal change in the presence of measurement error. Ann. Epidemiol. 2002, 12, 34–38. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Katz, R.; Kestenbaum, B.; Fried, L.F.; Siscovick, D.; Sarnak, M.J. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 2009, 204, 298–303. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek-Surdacka, E.; Świerszcz, J.; Surdacki, A. Effects of Atorvastatin Dose and Concomitant Use of Angiotensin-Converting Enzyme Inhibitors on Renal Function Changes over Time in Patients with Stable Coronary Artery Disease: A Prospective Observational Study. Int. J. Mol. Sci. 2016, 17, 106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020106
Wieczorek-Surdacka E, Świerszcz J, Surdacki A. Effects of Atorvastatin Dose and Concomitant Use of Angiotensin-Converting Enzyme Inhibitors on Renal Function Changes over Time in Patients with Stable Coronary Artery Disease: A Prospective Observational Study. International Journal of Molecular Sciences. 2016; 17(2):106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020106
Chicago/Turabian StyleWieczorek-Surdacka, Ewa, Jolanta Świerszcz, and Andrzej Surdacki. 2016. "Effects of Atorvastatin Dose and Concomitant Use of Angiotensin-Converting Enzyme Inhibitors on Renal Function Changes over Time in Patients with Stable Coronary Artery Disease: A Prospective Observational Study" International Journal of Molecular Sciences 17, no. 2: 106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17020106