Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Nile Tilapia (Oreochromis niloticus)
Abstract
:1. Introduction
2. Results
2.1. Identification of the Sox Genes in the Tilapia Genome
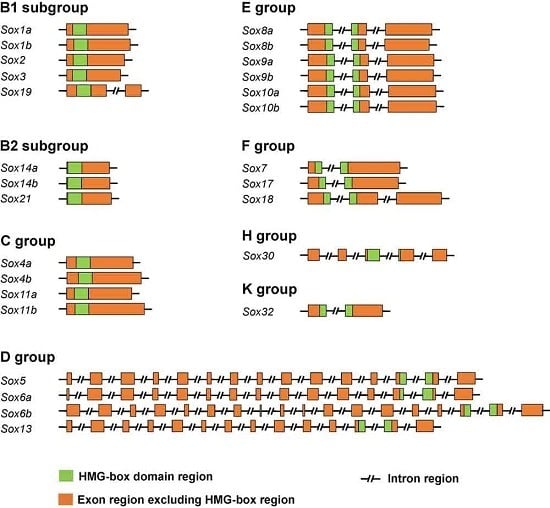
2.2. Genomic Structure of the Tilapia Sox Genes
2.3. Comparison of the Sox Genes Among the Tilapia and Other Animals
2.4. Spatial Expression of the Tilapia Sox Genes
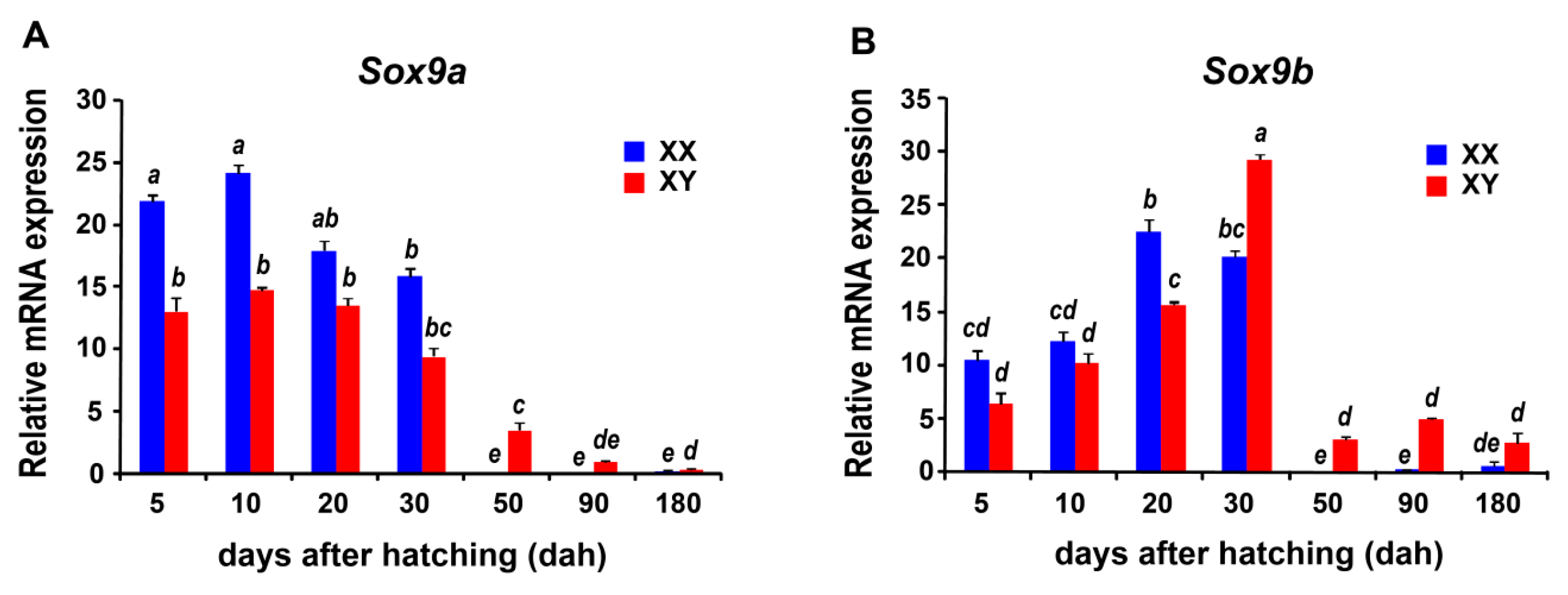
2.5. Temporal Expression of the Sox Genes in the Tilapia Gonads
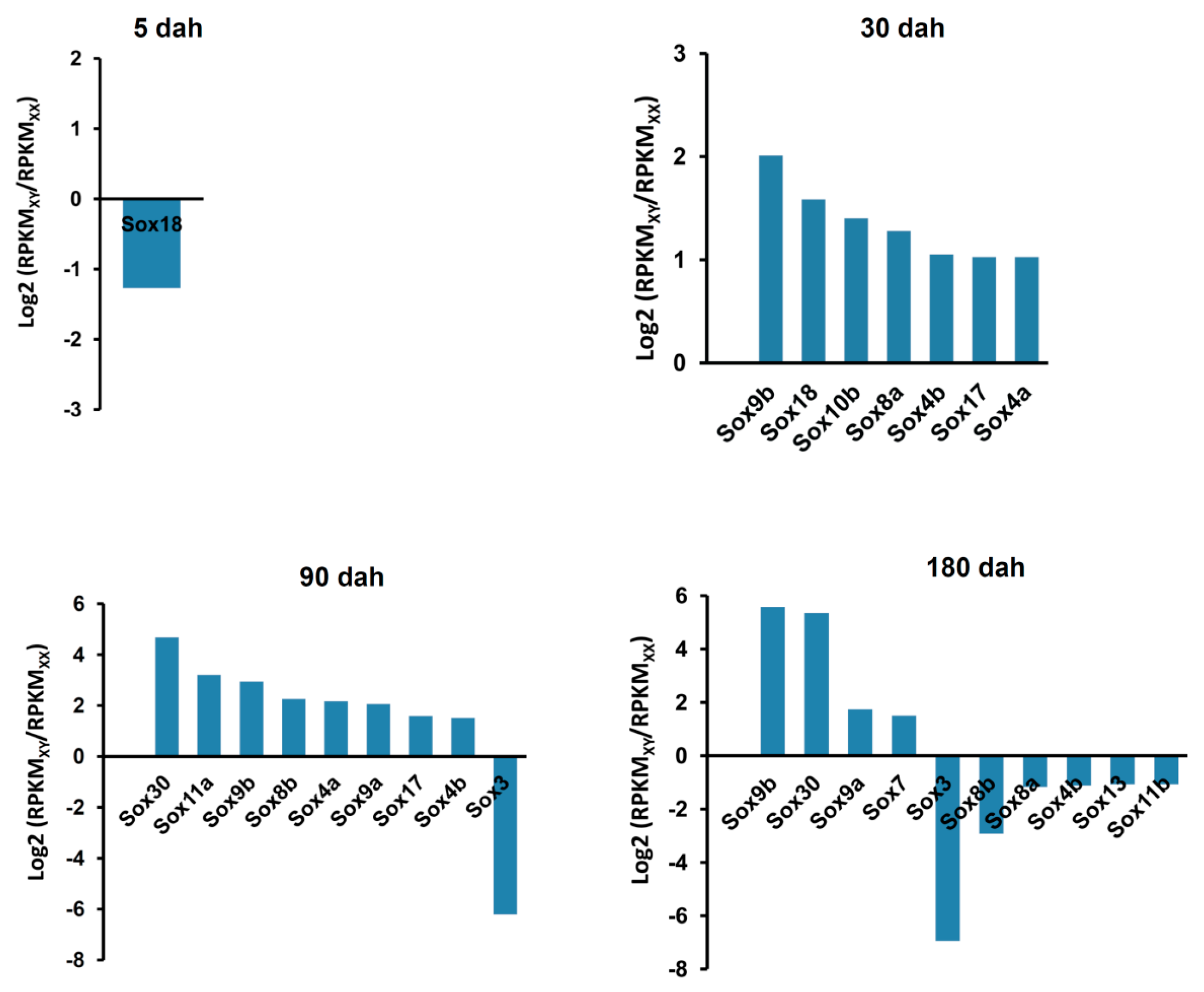
2.6. Sexually Dimorphic Expression of the Sox Genes in the Tilapia Gonads
3. Discussion
4. Materials and Methods
4.1. Animal Rearing
4.2. Genome-Wide Identification of the Sox Genes
4.3. Phylogenetic Analysis
4.4. Transcriptome-Based Analysis of Expression Profiling of the Sox Genes
4.5. Gene Expression Profiling by Quantitative RT-PCR
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HMG | high-mobility group |
| 1R | first round of genome duplication |
| 4R | fourth round of genome duplication |
| dah | days after hatching |
| RPKM | reads per kb per million read |
References
- Fortunato, S.; Adamski, M.; Bergum, B.; Guder, C.; Jordal, S.; Leininger, S.; Zwafink, C.; Rapp, H.T.; Adamska, M. Genome-wide analysis of the sox family in the calcareous sponge Sycon ciliatum: Multiple genes with unique expression patterns. EvoDevo 2012, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, X.; Zhao, H.; Nagahama, Y. Genome-wide analysis of Sox genes in Medaka (Oryzias latipes) and their expression pattern in embryonic development. Cytogenet. Genome Res. 2011, 134, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cremazy, F.; Berta, P.; Girard, F. Genome-wide analysis of Sox genes in Drosophila melanogaster. Mech. Dev. 2001, 109, 371–375. [Google Scholar] [CrossRef]
- Uy, B.R.; Simoes-Costa, M.; Sauka-Spengler, T.; Bronner, M.E. Expression of Sox family genes in early lamprey development. Int. J. Dev. Biol. 2012, 56, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Koopman, P.; Schepers, G.; Brenner, S.; Venkatesh, B. Origin and diversity of the Sox transcription factor gene family: Genome-wide analysis in Fugu rubripes. Gene 2004, 328, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Sekido, R.; Lovell-Badge, R. Sex determination and SRY: Down to a wink and a nudge? Trends Genet. 2009, 25, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Wheatley, S.C.; Andrews, J.E.; Sinclair, A.H.; Koopman, P. A male-specific role for SOX9 in vertebrate sex determination. Development 1996, 122, 2813–2822. [Google Scholar] [PubMed]
- Jiang, T.; Hou, C.C.; She, Z.Y.; Yang, W.X. The SOX gene family: Function and regulation in testis determination and male fertility maintenance. Mol. Biol. Rep. 2012, 40, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, V.G. SRY and SOX9: The main genetic factors of mammalian sex determination. Tsitologiia 2012, 54, 390–404. [Google Scholar] [PubMed]
- Jager, M.; Queinnec, E.; Houliston, E.; Manuel, M. Expansion of the SOX gene family predated the emergence of the Bilateria. Mol. Phylogenet. Evol. 2006, 39, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 2011, 25, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sakai, D.; Osumi, N.; Wada, H.; Wakamatsu, Y. Sox genes regulate type 2 collagen expression in avian neural crest cells. Dev. Growth Differ. 2006, 48, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Zhu, T.Y.; Lin, D.; Zhang, Y.; Zhang, W.M. A second form of Sox11 homologue identified in the orange-spotted grouper Epinephelus coioides: Analysis of sequence and mRNA expression patterns. Comp. Biochem. Phys. B 2010, 157, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.J.; Harley, V.R. Sex with two SOX on: SRY and SOX9 in testis development. Trends Endocrinol. Metab. 2002, 13, 106–111. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Scherer, G. SOX E genes: SOX9 and SOX8 in mammalian testis development. Int. J. Biochem. Cell Biol. 2010, 42, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Liu, S.J.; Tao, M.; Li, W.; Liu, Y. Isolation and expression analysis of testicular type Sox9b in allotetraploid fish. Mar. Biotechnol. 2007, 9, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Soriano, N.S.; Russell, S. The Drosophila SOX-domain protein Dichaete is required for the development of the central nervous system midline. Development 1998, 125, 3989–3996. [Google Scholar] [PubMed]
- Mukherjee, A.; Shan, X.; Mutsuddi, M.; Ma, Y.; Nambu, J.R. The Drosophila sox gene, fish-hook, is required for postembryonic development. Dev. Biol. 2000, 217, 91–106. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Krishnamurthy, M.; Goodyer, C.G.; Wang, R. The emerging role of SOX transcription factors in pancreatic endocrine cell development and function. Stem Cells Dev. 2009, 18, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Gracz, A.D.; Magness, S.T. Sry-box (Sox) transcription factors in gastrointestinal physiology and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G503–G515. [Google Scholar] [CrossRef] [PubMed]
- Lioubinski, O.; Muller, M.; Wegner, M.; Sander, M. Expression of Sox transcription factors in the developing mouse pancreas. Dev. Dyn. 2003, 227, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kamekura, S.; Mabuchi, A.; Kou, I.; Seki, S.; Takato, T.; Nakamura, K.; Kawaguchi, H.; Ikegawa, S.; Chung, U.I. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004, 50, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.L.; Willoughby, J.; Liu, D.; Crump, J.G.; Wilson, C.; Miller, C.T.; Singer, A.; Kimmel, C.; Westerfield, M.; Postlethwait, J.H. A pair of Sox: Distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 2005, 132, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Leveugle, M.; Prat, K.; Popovici, C.; Birnbaum, D.; Coulier, F. Phylogenetic analysis of Ciona intestinalis gene superfamilies supports the hypothesis of successive gene expansions. J. Mol. Evol. 2004, 58, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cheng, D.; Li, D.; Meng, M.; Peng, L.; Tang, L.; Pan, M.; Xiang, Z.; Xia, Q.; Lu, C. Identification and characterization of Sox genes in the silkworm, Bombyx mori. Mol. Biol. Rep. 2011, 38, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Howard-Ashby, M.; Materna, S.C.; Brown, C.T.; Chen, L.; Cameron, R.A.; Davidson, E.H. Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev. Biol. 2006, 300, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Magie, C.R.; Pang, K.; Martindale, M.Q. Genomic inventory and expression of Sox and Fox genes in the cnidarian Nematostella vectensis. Dev. Genes Evol. 2005, 215, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Schepers, G.E.; Teasdale, R.D.; Koopman, P. Twenty pairs of Sox: Extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. Cell 2002, 3, 167–170. [Google Scholar] [CrossRef]
- Brawand, D.; Wagner, C.E.; Li, Y.I.; Malinsky, M.; Keller, I.; Fan, S.; Simakov, O.; Ng, A.Y.; Lim, Z.W.; Bezault, E.; et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature 2014, 513, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Watakabe, I.; Nishimura, T.; Toyoda, A.; Taniguchi, Y.; Tanaka, M. Analysis of medaka sox9 orthologue reveals a conserved role in germ cell maintenance. PLoS ONE 2012, 7, e29982. [Google Scholar] [CrossRef] [PubMed]
- Herpers, R.; van de Kamp, E.; Duckers, H.J.; Schulte-Merker, S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ. Res. 2008, 102, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, S.; Moleri, S.; Cimbro, S.; Corti, P.; Del Giacco, L.; Amodeo, R.; Dejana, E.; Koopman, P.; Cotelli, F.; Beltrame, M. Sox18 and Sox7 play redundant roles in vascular development. Blood 2008, 111, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Argenton, F.; Giudici, S.; Deflorian, G.; Cimbro, S.; Cotelli, F.; Beltrame, M. Ectopic expression and knockdown of a zebrafish sox21 reveal its role as a transcriptional repressor in early development. Mech. Dev. 2004, 121, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Cnaani, A.; Lee, B.Y.; Ozouf-Costaz, C.; Bonillo, C.; Baroiller, J.F.; D’Cotta, H.; Kocher, T. Mapping of sox2 and sox14 in tilapia (Oreochromis spp.). Sex. Dev. 2007, 1, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, Z.; Wu, F.; Liu, Z.; Huang, B.; Wang, D. Characterization, phylogeny, alternative splicing and expression of Sox30 gene. BMC Mol. Biol. 2010, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yuan, J.; Zhou, L.; Sun, L.; Sun, Y.; Yang, S.; Li, M.; Zeng, S.; Huang, B.; Wang, D. Characterization of gonadal transcriptomes from Nile tilapia (Oreochromis niloticus) reveals differentially expressed genes. PLoS ONE 2013, 8, e63604. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.; Neuhauss, S.C. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, F.A. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. Biol. Sci. 2012, 279, 5048–5057. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. All purpose Sox: The many roles of Sox proteins in gene expression. Int. J. Biochem. Cell Biol. 2010, 42, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Larroux, C.; Luke, G.N.; Koopman, P.; Rokhsar, D.S.; Shimeld, S.M.; Degnan, B.M. Genesis and expansion of metazoan transcription factor gene classes. Mol. Biol. Evol. 2008, 25, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wen, L.; Li, K.; Liu, X.; Luo, B.; Chen, F.; Xie, D.; Kung, H.F. Comparative analysis of duplicated sox21 genes in zebrafish. Dev. Growth Differ. 2011, 53, 347–356. [Google Scholar] [CrossRef] [PubMed]
- De Martino, S.; Yan, Y.L.; Jowett, T.; Postlethwait, J.H.; Varga, Z.M.; Ashworth, A.; Austin, C.A. Expression of sox11 gene duplicates in zebrafish suggests the reciprocal loss of ancestral gene expression patterns in development. Dev. Dyn. 2000, 217, 279–292. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Agathon, A.; Alexander, J.; Thisse, C.; Waldron, S.; Yelon, D.; Thisse, B.; Stainier, D.Y. Casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Dumitriu, B.; Penzo-Mendez, A.; Han, Y.; Pallavi, B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 2007, 39, 2195–2214. [Google Scholar] [CrossRef] [PubMed]
- Abdelalim, E.M.; Emara, M.M.; Kolatkar, P.R. The SOX transcription factors as key players in pluripotent stem cells. Stem Cells Dev. 2014, 23, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Kallstrom, M.; Muhr, J. Sox21 promotes the progression of vertebrate neurogenesis. Nat. Neurosci. 2005, 8, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, M.; Kamachi, Y.; Kondoh, H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: Their expression during embryonic organogenesis of the chicken. Mech. Dev. 1999, 84, 103–120. [Google Scholar] [CrossRef]
- Ijiri, S.; Kaneko, H.; Kobayashi, T.; Wang, D.S.; Sakai, F.; Paul-Prasanth, B.; Nakamura, M.; Nagahama, Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol. Reprod. 2008, 78, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhou, L.; Wang, Y.; Xia, W.; Gui, J.F. Differential expression and dynamic changes of SOX3 during gametogenesis and sex reversal in protogynous hermaphroditic fish. J. Exp. Zool. Part A Ecol. Genet. Phys. 2007, 307, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Meeks, J.J.; Hurley, L.; Raverot, G.; Frassetto, A.; Jameson, J.L. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol. Cell. Biol. 2003, 23, 8084–8091. [Google Scholar] [CrossRef] [PubMed]
- Osaki, E.; Nishina, Y.; Inazawa, J.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Ohsugi, M.; Tezuka, T.; Yoshida, M.; Semba, K. Identification of a novel Sry-related gene and its germ cell-specific expression. Nucleic Acids Res. 1999, 27, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kobayashi, T.; Chang, X.T.; Nagahama, Y. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 1998, 281, 362–372. [Google Scholar] [CrossRef]
- Niimi, T.; Hayashi, Y.; Futaki, S.; Sekiguchi, K. SOX7 and SOX17 regulate the parietal endoderm-specific enhancer activity of mouse laminin alpha 1 gene. J. Biol. Chem. 2004, 279, 38055–38061. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Hayashi, Y.; Yamashita, M.; Yagi, K.; Bono, H.; Hayashizaki, Y.; Okazaki, Y.; Sekiguchi, K. Molecular basis of constitutive production of basement membrane components—Gene expression profiles of Engelbreth-Holm-Swarm tumor and F9 embryonal carcinoma cells. J. Biol. Chem. 2003, 278, 50691–50701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Li, M.; Cheng, Y.; Jiang, D.; Sun, L.; Tao, W.; Zhou, L.; Wang, Z.; Wang, D. Isolation of doublesex- and mab-3-related transcription factor 6 and its involvement in spermatogenesis in tilapia. Biol. Reprod. 2014, 91, 136. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef] [PubMed]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal x. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Tao, W.J.; Chen, J.L.; Sun, L.N.; Zhou, L.Y.; Song, Q.; Wang, D.S. Genome-wide identification, evolution and expression analysis of nuclear receptor superfamily in Nile tilapia, Oreochromis niloticus. Gene 2015, 569, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.; Komori, H.K.; LaMere, S.; Podshivalova, K.; Salomon, D.R. Finding the active genes in deep RNA-seq gene expression studies. BMC Genom. 2013, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Tsagaratou, A.; Aijo, T.; Lio, C.W.; Yue, X.; Huang, Y.; Jacobsen, S.E.; Lahdesmaki, H.; Rao, A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, E3306–E3315. [Google Scholar] [CrossRef] [PubMed]

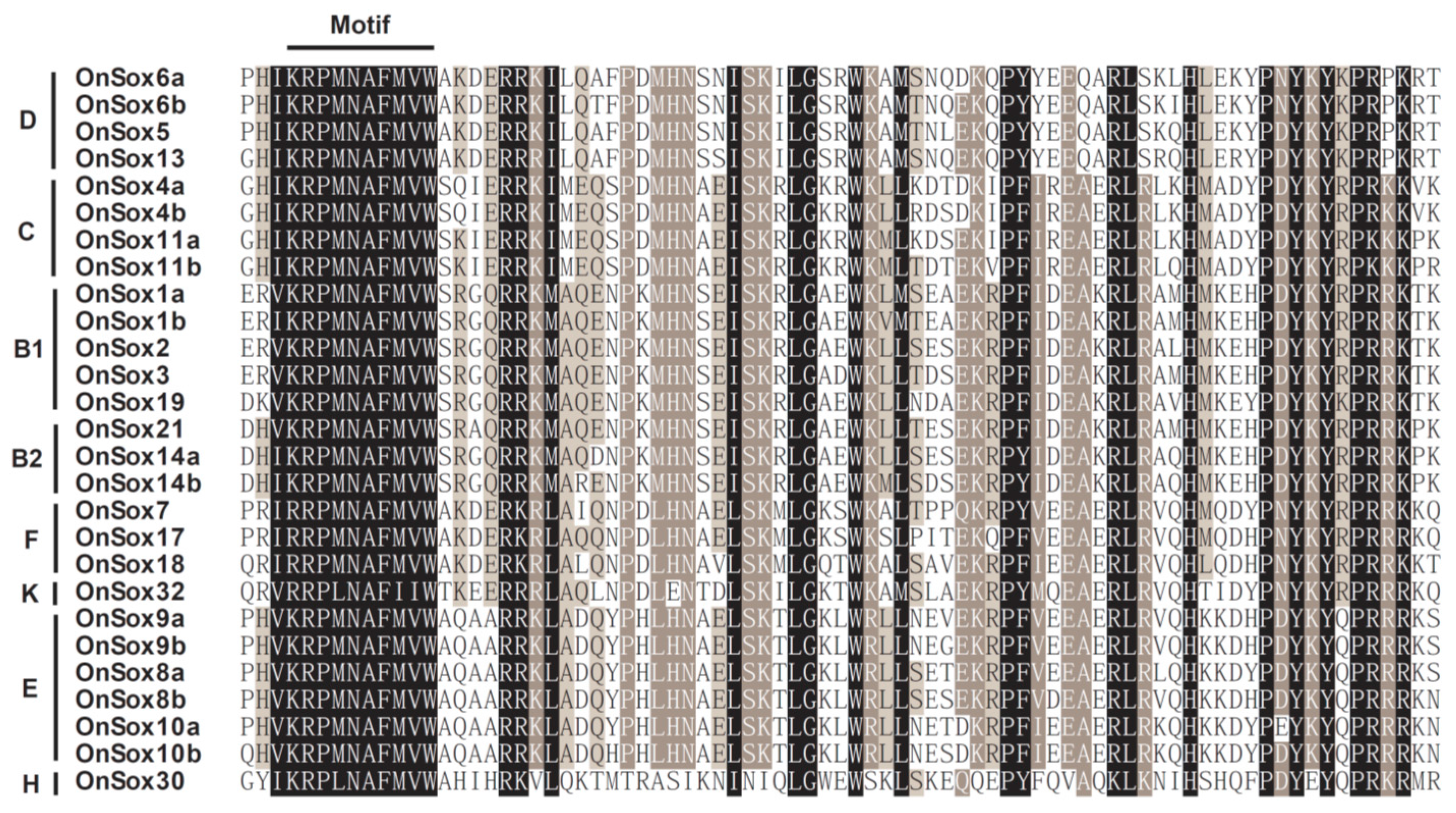

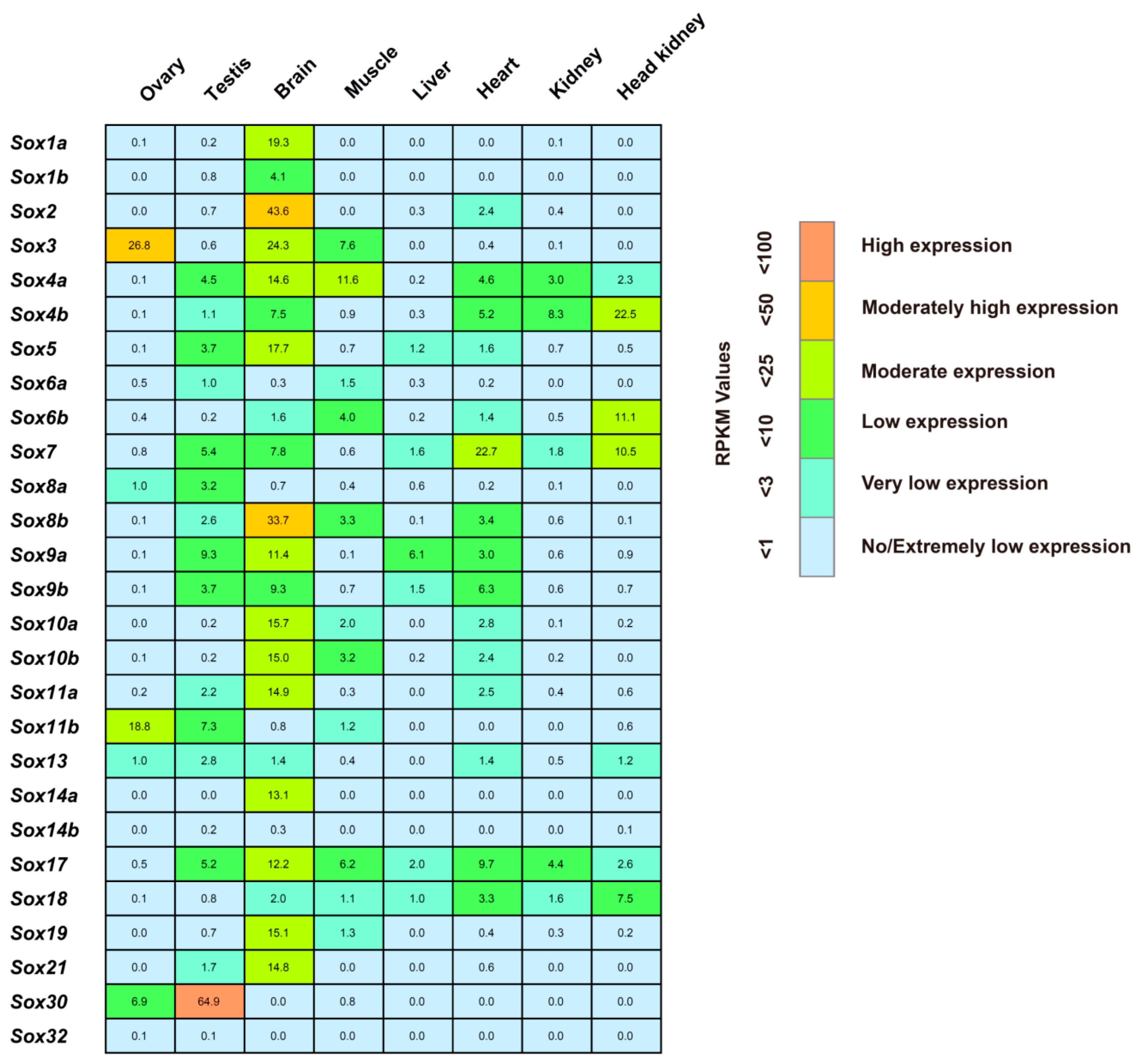
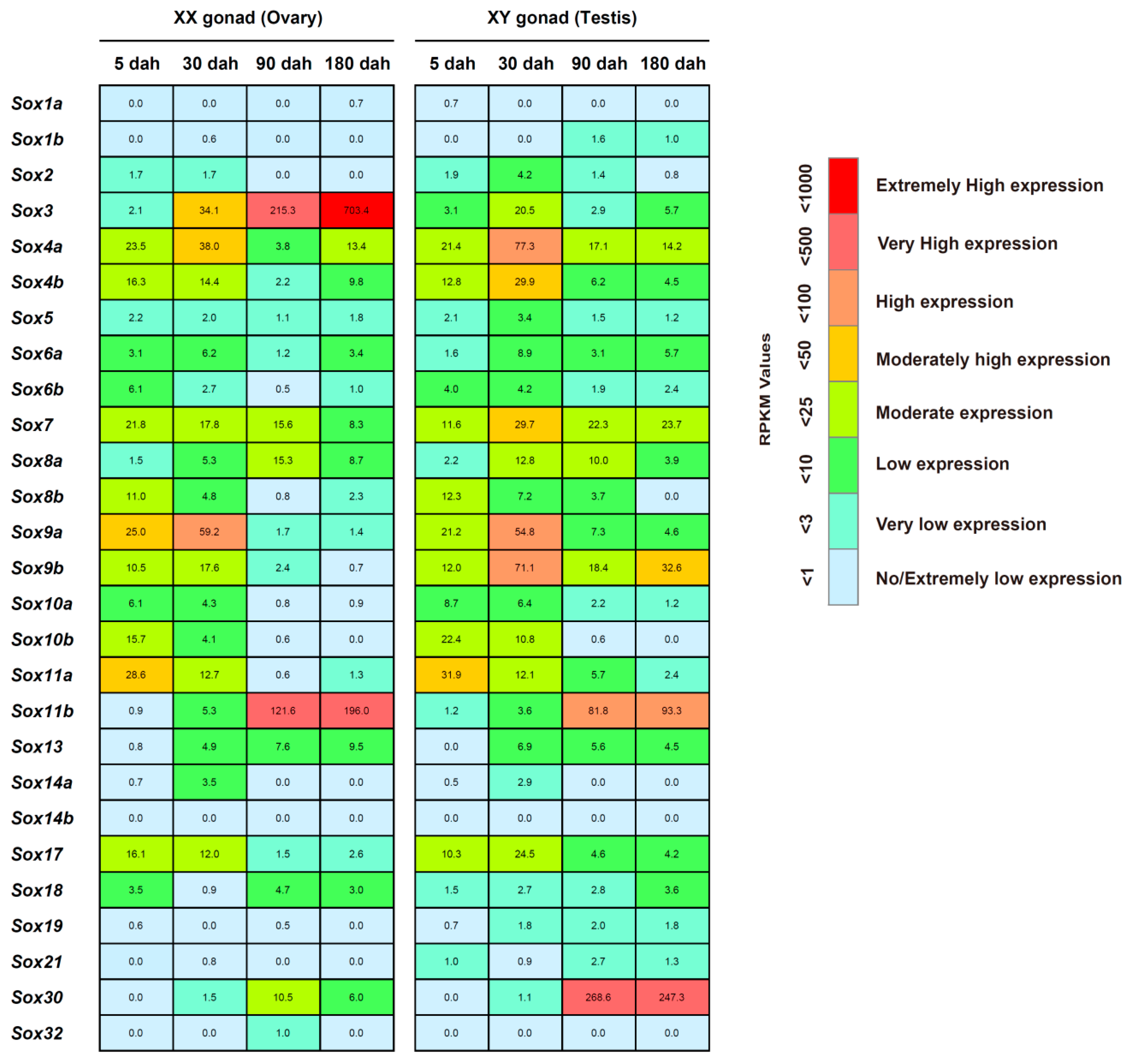
| Group | Gene | NCBI ID | Linkage Group | Position | Intron Number | Length (aa) | HMG-Box Position |
|---|---|---|---|---|---|---|---|
| B1 | Sox1a | XP_005457843.1 | 16–21 | 12,271,122–12,353,293 | 0 | 344 | 35–109 |
| Sox1b | XP_005450142.1 | unknown | 7,601,713–7,604,245 | 0 | 354 | 41–115 | |
| Sox2 | XP_003457401.1 | 17 | 280,838–283,149 | 0 | 322 | 38–112 | |
| Sox3 | XP_005467513.1 | 2 | 13,569,507–13,587,072 | 0 | 300 | 33–107 | |
| Sox19 | XP_003458966.1 | 3 | 282,331–286,532 | 1 | 307 | 57–131 | |
| B2 | Sox14a | XP_005451233.1 | 23 | 7,181,057–7,183,304 | 0 | 238 | 6–80 |
| Sox14b | XP_003438076.1 | 18 | 5,299,795–5,300,529 | 0 | 241 | 6–80 | |
| Sox21 | XP_003447333.1 | 16–21 | 14,804,010–14,806,286 | 0 | 248 | 6–80 | |
| C | Sox4a | XP_005450837.1 | 22 | 19,810,503–19,814,320 | 0 | 371 | 55–129 |
| Sox4b | XP_005450601.1 | 11 | 10,635,379–10,640,355 | 0 | 414 | 65–139 | |
| Sox11a | AAR01937.1 | 19 | 1,462,641–1,463,732 | 0 | 363 | 44–118 | |
| Sox11b | XP_005452537.1 | 15 | 7,943,145–7,945,227 | 0 | 433 | 48–122 | |
| D | Sox5 | XP_005451840.1 | 17 | 29,053,787–29,297,905 | 15 | 773 | 566–640 |
| Sox6a | XP_003442343.2 | 1 | 665,105–766,767 | 14 | 778 | 568–646 | |
| Sox6b | XP_005460016.1 | 7 | 667,787–732,752 | 17 | 838 | 613–691 | |
| Sox13 | XP_005450158.1 | 5 | 6,796,676–6,846,010 | 15 | 664 | 461–535 | |
| E | Sox8a | XP_003438722.1 | 8–24 | 6,051,795–6,054,972 | 2 | 479 | 96–170 |
| Sox8b | XP_003450163.1 | unknown | 1,216,205–1,219,881 | 2 | 464 | 98–172 | |
| Sox9a | XP_005448042.1 | 8–24 | 5,686,065–5,688,370 | 2 | 500 | 105–179 | |
| Sox9b | XP_003450167.1 | unknown | 1,462,602–1,466,515 | 2 | 484 | 102–176 | |
| Sox10a | XP_003447925.1 | 6 | 19,531,528–19,536,616 | 2 | 500 | 107–181 | |
| Sox10b | XP_005468484.1 | 4 | 7,506,891–7,515,424 | 2 | 503 | 105–179 | |
| F | Sox7 | XP_005460371.1 | 15 | 17,570,516–17,573,991 | 1 | 407 | 41–115 |
| Sox17 | XP_003439290.1 | 9 | 14,405,231–14,407,376 | 1 | 397 | 63–137 | |
| Sox18 | XP_003459774.1 | unknown | 18,060–22,872 | 2 | 565 | 90–164 | |
| H | Sox30 | XP_003447014.1 | unknown | 3,119,307–3,123,369 | 4 | 353 | 122–196 |
| K | Sox32 | XP_003439409.1 | 9 | 3,127,363–3,128,440 | 1 | 310 | 35–109 |
| Group | Common Carp (4R) | Nile Tilapia (3R) | Zebrafish (3R) | Pufferfish (3R) | Medaka (3R) | Human (2R) | Western Clawed (2R) | Chicken (2R) | Florida Lancelet (1R) | Fruit Fly |
|---|---|---|---|---|---|---|---|---|---|---|
| A | - | - | - | - | - | 1 | - | - | - | |
| B1 | 12 | 5 | 6 | 5 | 3 | 3 | 3 | 3 | 3 | 1 |
| B2 | 8 | 3 | 4 | 3 | 2 | 2 | 2 | 2 | 2 | 3 |
| C | 8 | 4 | 5 | 3 | 2 | 3 | 2 | 3 | 1 | 1 |
| D | 3 | 4 | 3 | 4 | 4 | 3 | 3 | 3 | 1 | 1 |
| E | 10 | 6 | 5 | 6 | 4 | 3 | 3 | 3 | 1 | 1 |
| F | 6 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 1 | 1 |
| G | - | - | - | - | - | 1 | - | - | - | - |
| H | - | 1 | - | - | - | 1 | - | 1 | 1 | - |
| I | - | - | - | - | - | - | 1 | - | - | - |
| J | - | - | - | - | - | - | - | - | - | - |
| K | 2 | 1 | 1 | 1 | 1 | - | - | - | - | - |
| Total | 49 | 27 | 27 | 25 | 19 | 20 | 18 | 18 | 10 | 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Yang, C.; Tao, W.; Wang, D. Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Nile Tilapia (Oreochromis niloticus). Int. J. Mol. Sci. 2016, 17, 270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030270
Wei L, Yang C, Tao W, Wang D. Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Nile Tilapia (Oreochromis niloticus). International Journal of Molecular Sciences. 2016; 17(3):270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030270
Chicago/Turabian StyleWei, Ling, Chao Yang, Wenjing Tao, and Deshou Wang. 2016. "Genome-Wide Identification and Transcriptome-Based Expression Profiling of the Sox Gene Family in the Nile Tilapia (Oreochromis niloticus)" International Journal of Molecular Sciences 17, no. 3: 270. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030270