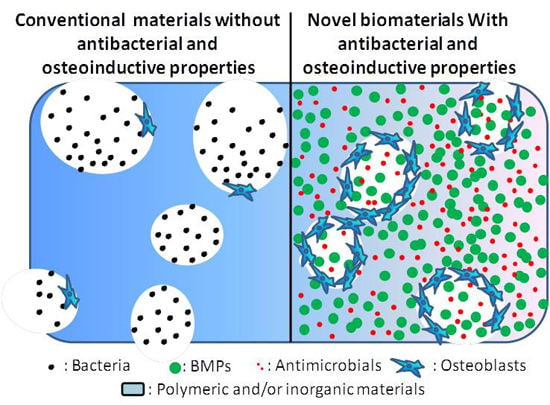
Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects
Abstract
:1. Introduction
1.1. Infected Bone Defects
1.2. Current Clinical Treatments
1.3. Novel Biomaterials with Both Antibacterial and Osteoinductive Properties
2. Osteoinductive Agents and Osteogenic Activities
2.1. BMPs
2.2. The Signaling Pathways and Osteoinductivity of BMPs
2.3. Clinical Applications of BMPs
2.4. BMPs with a Higher Osteoinductive Efficiency
3. Antimicrobials
3.1. Antibiotics
3.1.1. Tetracyclines
3.1.2. Vancomycin
3.1.3. Tobramycin
3.1.4. Effect of Antibiotics on in Vitro Osteogenic Activities
3.2. Antimicrobial Biomaterials
3.2.1. AgNPs
3.2.2. Qaternised Chitosan
4. Co-Delivery Systems for Antibacterial and Osteoinductive Drugs to Repair Infected Bone Defects
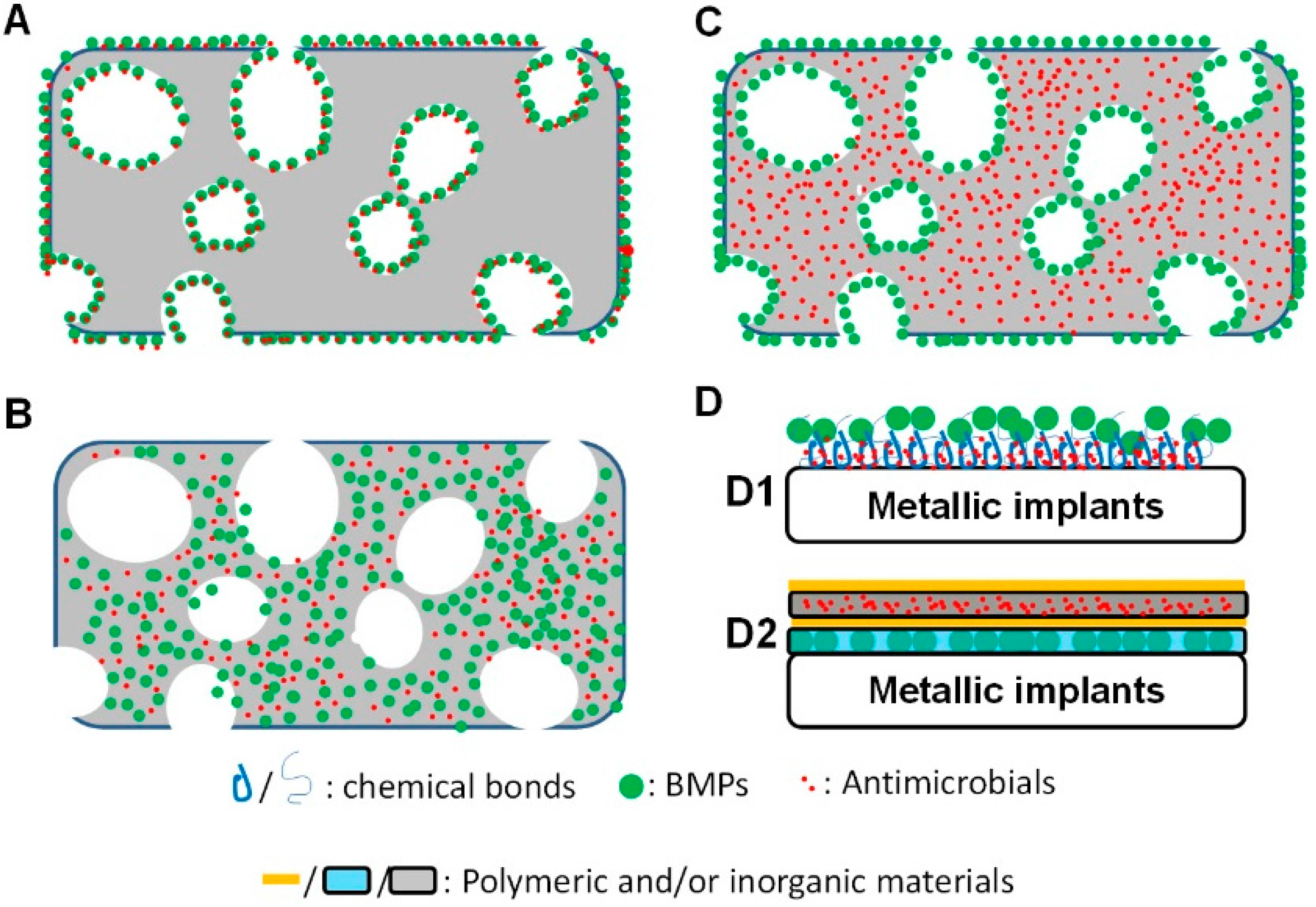
4.1. Adsorption and Physicochemical Bonds
4.2. Co-Encapsulation for a Simultaneous Release
4.3. A Mixed Carrying Mode for a Sequential Release
4.4. Surface Coatings
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 299–308. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mundell, R.D.; Mooney, M.P.; Siegel, M.I.; Losken, A. Osseous guided tissue regeneration using a collagen barrier membrane. J. Oral Maxillofac. Surg. 1993, 51, 1004–1012. [Google Scholar] [CrossRef]
- Stetzer, K.; Cooper, G.; Gassner, R.; Kapucu, R.; Mundell, R.; Mooney, M.P. Effects of fixation type and guided tissue regeneration on maxillary osteotomy healing in rabbits. J. Oral Maxillofac. Surg. 2002, 60, 427–436. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Injuries and Violence: The Facts 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Binkowska, A.M.; Michalak, G.; Slotwinski, R. Current views on the mechanisms of immune responses to trauma and infection. Cent. Eur. J. Immunol. 2015, 40, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Ellington, J.K.; Harris, M.; Webb, L.; Smith, B.; Smith, T.; Tan, K.; Hudson, M. Intracellular Staphylococcus aureus: A mechanism for the indolence of osteomyelitis. J. Bone Jt. Surg. Br. 2003, 85, 918–921. [Google Scholar]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDS) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A., Jr.; Bumgardner, J.D. Biomaterial and antibiotic strategies for peri-implantitis: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, I.; Berglundh, T.; Marinello, C.; Liljenberg, B.; Lindhe, J. Long-standing plaque and gingivitis at implants and teeth in the dog. Clin. Oral Implants Res. 1992, 3, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Berglundh, T.; Ericsson, I.; Liljenberg, B.; Marinello, C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin. Oral Implants Res. 1992, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schou, S.; Holmstrup, P.; Stoltze, K.; Hjorting-Hansen, E.; Kornman, K.S. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth. Clin. Oral Implants Res. 1993, 4, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. Peri-implantitis. Periodontology 2000 2010, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Boer, L.; Jaspers, V.; van der Loos, C.M.; van Wamel, W.J.; Wu, G.; Kwakman, P.H.; Zaat, S.A. Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 2014, 10, 5202–5212. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.S.; Bostrom, M.P. Synthetic bone scaffolds and fracture repair. Injury 2007, 38, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Heary, R.F.; Schlenk, R.P.; Sacchieri, T.A.; Barone, D.; Brotea, C. Persistent iliac crest donor site pain: Independent outcome assessment. Neurosurgery 2002, 50, 510–516. [Google Scholar] [PubMed]
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003, 28, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Abramson, M. Membranous vs. endochondrial bone autografts. Arch. Otolaryngol. 1974, 99, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Vuyk, H.D.; Adamson, P.A. Biomaterials in rhinoplasty. Clin. Otolaryngol. Allied Sci. 1998, 23, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ayerza, M.A.; Aponte-Tinao, L.A.; Abalo, E.; Muscolo, D.L. Continuity and function of patellar tendon host-donor suture in tibial allograft. Clin. Orthop. Relat. Res. 2006, 450, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, D.L.; Ayerza, M.A.; Aponte-Tinao, L.A.; Ranalletta, M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J. Bone Jt. Surg. Am. 2006, 88, 305–321. [Google Scholar] [CrossRef]
- Buck, B.E.; Malinin, T.I.; Brown, M.D. Bone transplantation and human immunodeficiency virus: An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin. Orthop. Relat. Res. 1989, 129–136. [Google Scholar] [CrossRef]
- Moreau, M.F.; Gallois, Y.; Basle, M.F.; Chappard, D. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials 2000, 21, 369–376. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Rebmann, V.; Passler, M.; Schollmeier, G.; Ekkernkamp, A.; Grosse-Wilde, H.; Tomford, W.W. Immune response to perforated and partially demineralized bone allografts. J. Orthop. Sci. 2001, 6, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Glauser, R.; Scharer, P.; Hammerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP2 on guided bone regeneration in humans. Clin. Oral Implants Res. 2003, 14, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Milovancev, M.; Muir, P.; Manley, P.A.; Seeherman, H.J.; Schaefer, S. Clinical application of recombinant human bone morphogenetic protein-2 in 4 dogs. Vet. Surg. 2007, 36, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Filion, T.M.; Ayers, D.C.; Song, J. pHEMA-nHA encapsulation and delivery of vancomycin and rhBMP-2 enhances its role as a bone graft substitute. Clin. Orthop. Relat. Res. 2013, 471, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, H.; Xue, D.; Shi, Z.; Qi, Y.; Ma, Q.; Pan, Z. Assessing the character of the rhBMP-2- and vancomycin-loaded calcium sulphate composites in vitro and in vivo. Arch. Orthop. Trauma Surg. 2011, 131, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yin, W.; Zara, J.N.; Li, W.; Kwak, J.; Mamidi, R.; Lee, M.; Siu, R.K.; Ngo, R.; Wang, J.; et al. The use of BMP-2 coupled—Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 2010, 31, 9293–9300. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, J.; Liu, J.; Wei, L.; Wu, G. BMP-functionalised coatings to promote osteogenesis for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 10150–10168. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. BMPs: From bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005, 16, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from the laboratory to the clinic, part i (basic concepts). J. Tissue Eng. Regen. Med. 2008, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.X.; Moore, R.K.; Otsuka, F.; Shimasaki, S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9: Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J. Biol. Chem. 2003, 278, 3713–3719. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in bmp receptor signaling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (BMPs): From morphogens to metabologens. Cytokine Growth Factor Rev. 2009, 20, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; Hyun, J.S.; Nelson, E.R.; Li, S.; Montoro, D.T.; Wan, D.C.; Jia, F.J.; Glotzbach, J.C.; James, A.W.; Lee, M.; et al. Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cells 2011, 29, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Im, G.I. Combination of transforming growth factor-β2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng. A 2009, 15, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Gopalakrishnan, R.; Jiang, D.; Reith, E.; Benson, M.D.; Franceschi, R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-e1 cells. J. Bone Miner. Res. 2002, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, G.; Zhao, J.; Wang, L.; Sun, P.; Gu, Z. RhBMP2/7 heterodimer: An osteoblastogenesis inducer of not higher potency but lower effective concentration compared with rhBMP2 and rhBMP7 homodimers. Tissue Eng. A 2010, 16, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Ferrari, D.; Sager, M.; Becker, J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhgdf-5 and rhBMP2: An immunohistochemical study in dogs. Clin. Oral Implants Res. 2008, 19, 642–652. [Google Scholar] [PubMed]
- Shields, L.B.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Cooper, G.M.; Mooney, M.P.; Marra, K.G.; Losee, J.E. Bone morphogenetic protein 2 therapy for craniofacial surgery. J. Craniofac. Surg. 2008, 19, 1244–1259. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Boden, S.D.; Burkus, J.K.; Badura, J.M.; Peckham, S.M.; McKay, W.F. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine 2009, 34, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S. Controlled release scaffolds for bone tissue engineering. J. Pharm. Sci. 2008, 98, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, J.; Liu, T.; Gao, L.; Gu, Z.; Wu, G. Low-dose rhBMP2/7 heterodimer to reconstruct peri-implant bone defects: A micro-ct evaluation. J. Clin. Periodontol. 2012, 39, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tang, M.; Huang, J.; He, B.C.; Gao, J.L.; Chen, L.; Zuo, G.W.; Zhang, W.; Luo, Q.; Shi, Q.; et al. TGFβ/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J. Biol. Chem. 2010, 285, 29588–29598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, S.; Li, M.; Zhang, J.; Bi, Y.; He, Y.; Liu, X.; Nan, G.; Su, Y.; Zhu, G.; et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J. Orthop. Res. 2013, 31, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schmidt, A.H.; Mahjouri, S.; Polly, D.W., Jr.; Lew, W.D. Union of a chronically infected internally stabilized segmental defect in the rat femur after debridement and application of rhBMP2 and systemic antibiotic. J. Orthop. Trauma 2007, 21, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.S.; Supronowicz, P.R.; Zhukauskas, R.M.; Gill, E.; Cobb, R.R. Local antibiotic delivery with demineralized bone matrix. Cell Tissue Bank. 2012, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Howe, T.G. Bacterial resistance to the tetracyclines. Microbiol. Rev. 1978, 42, 707–724. [Google Scholar] [PubMed]
- Suzuki, A.; Yagisawa, J.; Kumakura, S.; Tsutsui, T. Effects of minocycline and doxycycline on cell survival and gene expression in human gingival and periodontal ligament cells. J. Periodontal Res. 2006, 41, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sorensen, W.P.; Wang, H.L. Management and prevention of retrograde peri-implant infection from retained root tips: Two case reports. Int. J. Periodontics Restorative Dent. 2004, 24, 422–433. [Google Scholar] [PubMed]
- Golub, L.M.; Ramamurthy, N.; McNamara, T.F.; Gomes, B.; Wolff, M.; Casino, A.; Kapoor, A.; Zambon, J.; Ciancio, S.; Schneir, M.; et al. Tetracyclines inhibit tissue collagenase activity. A new mechanism in the treatment of periodontal disease. J. Periodontal Res. 1984, 19, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Evans, R.T.; Coburn, R.A.; Genco, R.J. Tetracycline and its derivatives strongly bind to and are released from the tooth surface in active form. J. Periodontol. 1983, 54, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Heasman, P.A. Tetracyclines in the management of periodontal diseases: A review. J. Clin. Periodontol. 1995, 22, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.P. Vancomycin: A history. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Kotra, L.P.; Haddad, J.; Mobashery, S. Aminoglycosides: Perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 2000, 44, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987, 51, 341–350. [Google Scholar] [PubMed]
- Park, J.B. Effects of doxycycline, minocycline, and tetracycline on cell proliferation, differentiation, and protein expression in osteoprecursor cells. J. Craniofac. Surg. 2011, 22, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Almazin, S.M.; Dziak, R.; Andreana, S.; Ciancio, S.G. The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J. Periodontol. 2009, 80, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Low dose of doxycyline promotes early differentiation of preosteoblasts by partially regulating the expression of estrogen receptors. J. Surg. Res. 2012, 178, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Muthukuru, M.; Sun, J. Doxycycline counteracts bone morphogenic protein 2-induced osteogenic mediators. J. Periodontol. 2013, 84, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Alzohairy, M.A. Anti-biofilm efficacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J. Med. Microbiol. 2015, 33, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nunez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.M.; Herrera, J.L.; Fernandez-Montesinos, R.; Caro, C.; Zaderenko, A.P.; Mejias, J.A.; Pozo, D. Tiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by toll-like receptor ligands. Nanomedicine 2008, 3, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Boucher, W.; Stern, J.M.; Kotsinyan, V.; Kempuraj, D.; Papaliodis, D.; Cohen, M.S.; Theoharides, T.C. Intravesical nanocrystalline silver decreases experimental bladder inflammation. J. Urol. 2008, 179, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitis. J. Inflamm. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Lackner, P.; Beer, R.; Broessner, G.; Helbok, R.; Galiano, K.; Pleifer, C.; Pfausler, B.; Brenneis, C.; Huck, C.; Engelhardt, K.; et al. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit. Care 2008, 8, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Huang, C.Y.; Chuang, S.S.; Chen, C.C. A clinical experience of treating exfoliative wounds using nanocrystalline silver-containing dressings (acticoat). Burns 2007, 33, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.H.; Chen, C.F.; Huang, S.; Shih, T.S.; Lai, P.S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.; Li, Z.; Casciano, D.; Khodakovskaya, M.V.; Chen, T.; Karmakar, A.; Dervishi, E.; Xu, Y.; Mustafa, T.; Watanabe, F.; et al. Nanostructural materials increase mineralization in bone cells and affect gene expression through mirna regulation. J. Cell. Mol. Med. 2011, 15, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.X.; Wang, L.; Du, L.; Guo, S.R.; Wang, X.Q.; Tang, T.T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Daly, W.H. Quaternization of N-aryl chitosan derivatives: Synthesis, characterization, and antibacterial activity. Carbohydr. Res. 2009, 344, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Jarmila, V.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [PubMed]
- Kenawy el, R.; Abdel-Hay, F.I.; el-Raheem, A.; el-Shanshoury, R.; el-Newehy, M.H. Biologically active polymers: Synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphonium groups. J. Control. Release 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Park, D.K.; Kim, S.S.; Thakur, N.; Boden, S.D. Use of recombinant human bone morphogenetic protein-2 with local bone graft instead of iliac crest bone graft in posterolateral lumbar spine arthrodesis. Spine 2013, 38, E738–E747. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, H.; Vedantham, K.; Aniket; Young, A.; Marriott, I.; El-Ghannam, A. Tissue engineering scaffold for sequential release of vancomycin and rhBMP2 to treat bone infections. J. Biomed. Mater. Res. A 2014, 102, 4213–4223. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Sun, C.Y.; Che, Y.J.; Lu, S.J. Preparation and application of collagen scaffold-encapsulated silver nanoparticles and bone morphogenetic protein 2 for enhancing the repair of infected bone. Biotechnol. Lett. 2015, 37, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Chen, J.; Gao, F.; Brydson, R.; Johnson, B.; Heath, G.; Zhang, Y.; Wu, L.; Zhou, D. Near-infrared fluorescent ribonuclease-a-encapsulated gold nanoclusters: Preparation, characterization, cancer targeting and imaging. Nanoscale 2013, 5, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Kanellakopoulou, K.; Galanopoulos, I.; Soranoglou, V.; Tsaganos, T.; Tziortzioti, V.; Maris, I.; Papalois, A.; Giamarellou, H.; Giamarellos-Bourboulis, E.J. Treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with a synthetic carrier of calcium sulphate (stimulan) releasing moxifloxacin. Int. J. Antimicrob. Agents 2009, 33, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sanicola, S.M.; Albert, S.F. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J. Foot Ankle Surg. 2005, 44, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Doty, H.A.; Leedy, M.R.; Courtney, H.S.; Haggard, W.O.; Bumgardner, J.D. Composite chitosan and calcium sulfate scaffold for dual delivery of vancomycin and recombinant human bone morphogenetic protein-2. J. Mater. Sci. Mater. Med. 2014, 25, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Guelcher, S.A.; Brown, K.V.; Li, B.; Guda, T.; Lee, B.H.; Wenke, J.C. Dual-purpose bone grafts improve healing and reduce infection. J. Orthop. Trauma 2011, 25, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, A.E.; Li, B.; Yoshii, T.; Zienkiewicz, K.; Davidson, J.M.; Guelcher, S.A. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm. Res. 2008, 25, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, J.; Filion, T.; Saiz, E.; Tomsia, A.P.; Lian, J.B.; Stein, G.S.; Ayers, D.C.; Bertozzi, C.R. Elastomeric high-mineral content hydrogel-hydroxyapatite composites for orthopedic applications. J. Biomed. Mater. Res. A 2009, 89, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Skelly, J.D.; Lange, J.; Filion, T.M.; Li, X.; Ayers, D.C.; Song, J. Vancomycin-bearing synthetic bone graft delivers rhBMP2 and promotes healing of critical rat femoral segmental defects. Clin. Orthop. Relat. Res. 2014, 472, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Lian, J.B.; Ayers, D.C.; Song, J. Sustained and localized in vitro release of BMP2/7, RANKL, and tetracycline from flexbone, an elastomeric osteoconductive bone substitute. J. Orthop. Res. 2009, 27, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP2) or three (gentamicin, IGF-I and BMP2) substances from a one-component polymeric coating on implants. J. Control. Release 2011, 156, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Guo, H.; Lu, J.; Shi, J.; Wei, J.; Liu, C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP2 by synchrotron radiation-based muct. Biomaterials 2011, 32, 8506–8517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, Y.; Cheng, X.; Wang, P.; Xie, Y.; Xu, S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP2. Biomaterials 2014, 35, 10033–10045. [Google Scholar] [CrossRef] [PubMed]
- Vlacic-Zischke, J.; Hamlet, S.M.; Friis, T.; Tonetti, M.S.; Ivanovski, S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFβ/BMP signalling in osteoblasts. Biomaterials 2011, 32, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: The surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng. C Methods 2010, 16, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. The effect of a slow mode of BMP2 delivery on the inflammatory response provoked by bone-defect-filling polymeric scaffolds. Biomaterials 2010, 31, 7485–7493. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
 : clarified mechanisms;
: clarified mechanisms;  : unclarified mechanisms.
: unclarified mechanisms.
 : clarified mechanisms;
: clarified mechanisms;  : unclarified mechanisms.
: unclarified mechanisms.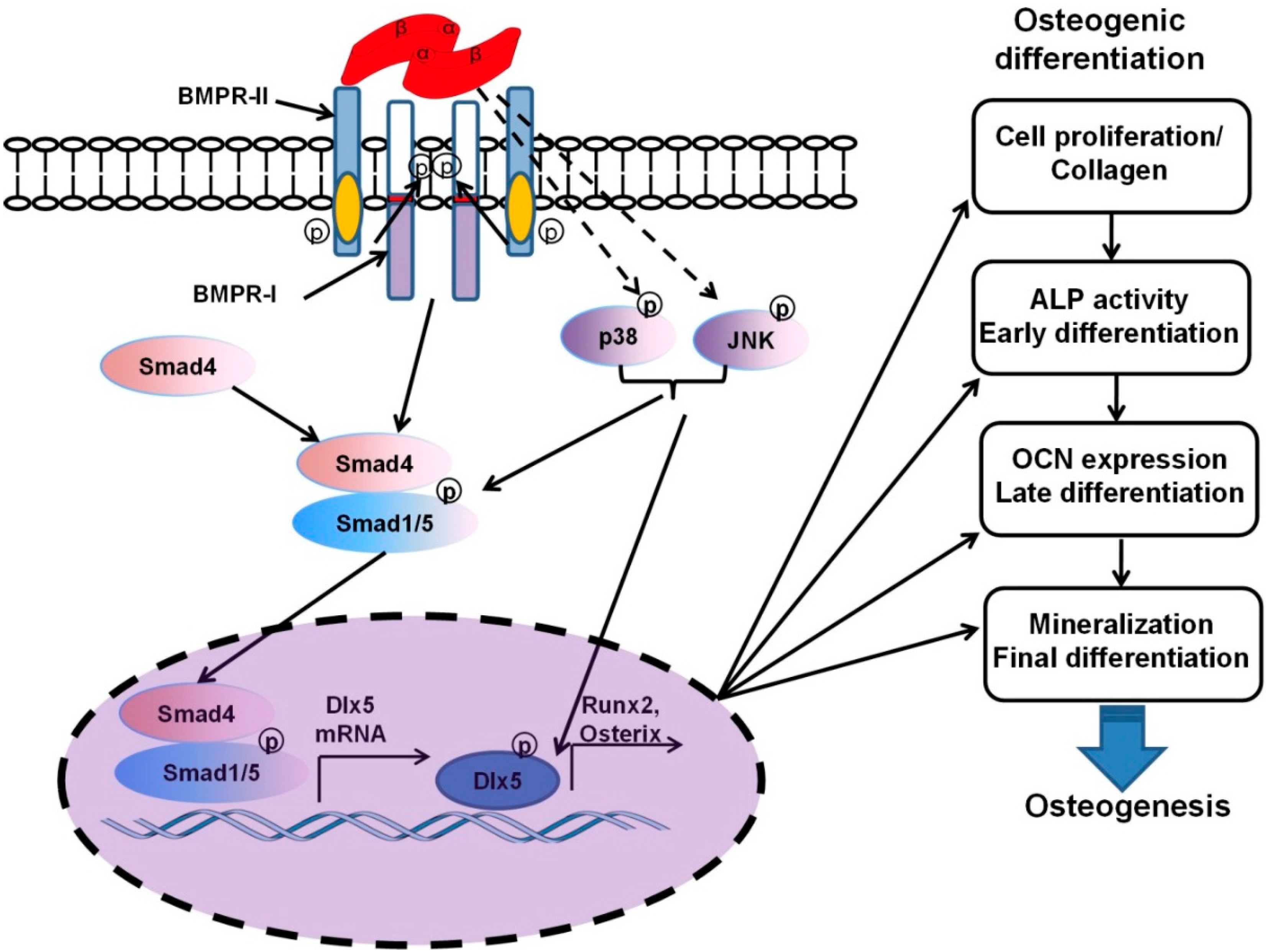
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030334
Lu H, Liu Y, Guo J, Wu H, Wang J, Wu G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. International Journal of Molecular Sciences. 2016; 17(3):334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030334
Chicago/Turabian StyleLu, Haiping, Yi Liu, Jing Guo, Huiling Wu, Jingxiao Wang, and Gang Wu. 2016. "Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects" International Journal of Molecular Sciences 17, no. 3: 334. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030334