Dried Saliva Spots: A Robust Method for Detecting Streptococcus pneumoniae Carriage by PCR
Abstract
:1. Introduction
2. Results
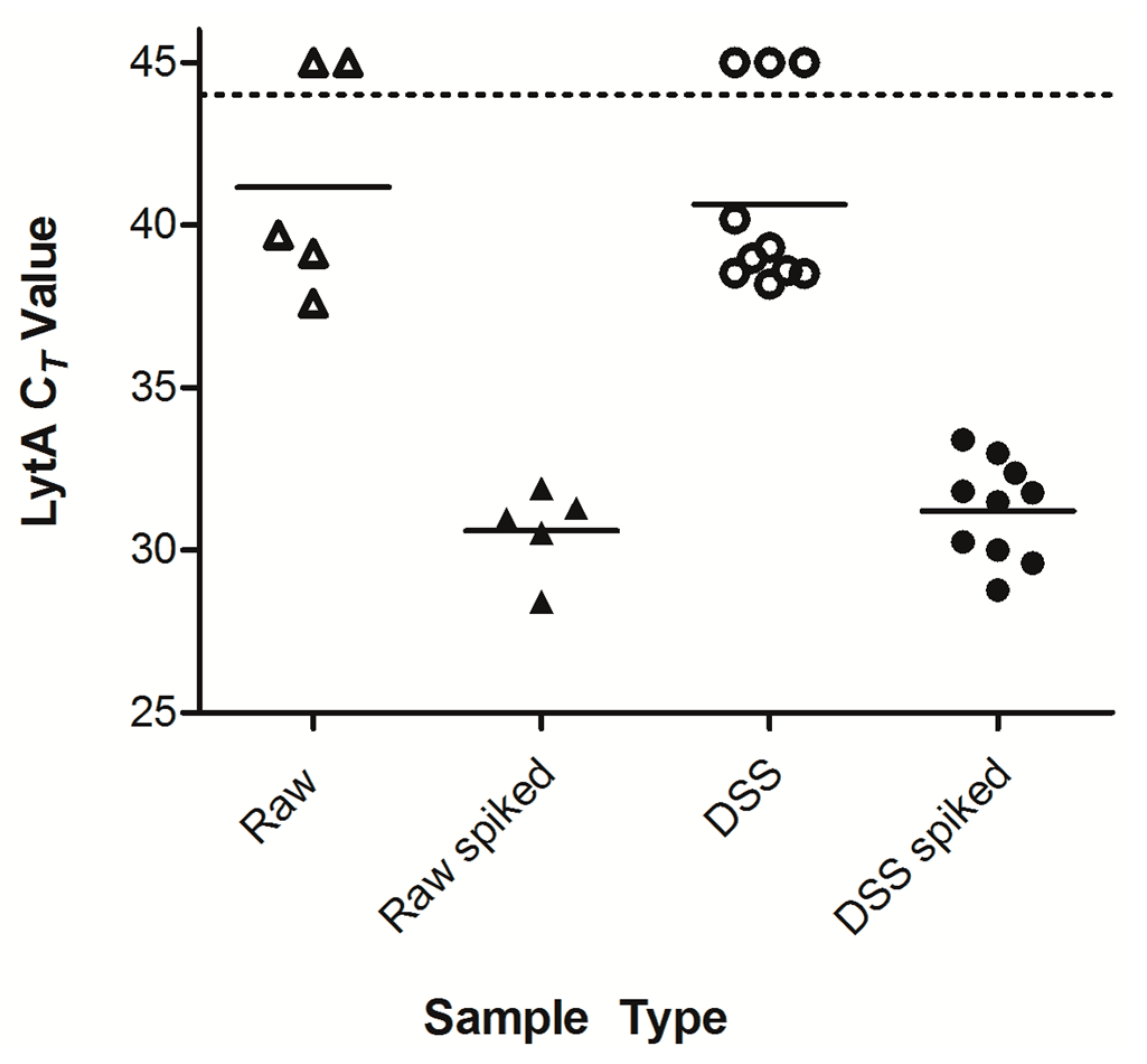
2.1. Optimization of Dried Saliva Spots
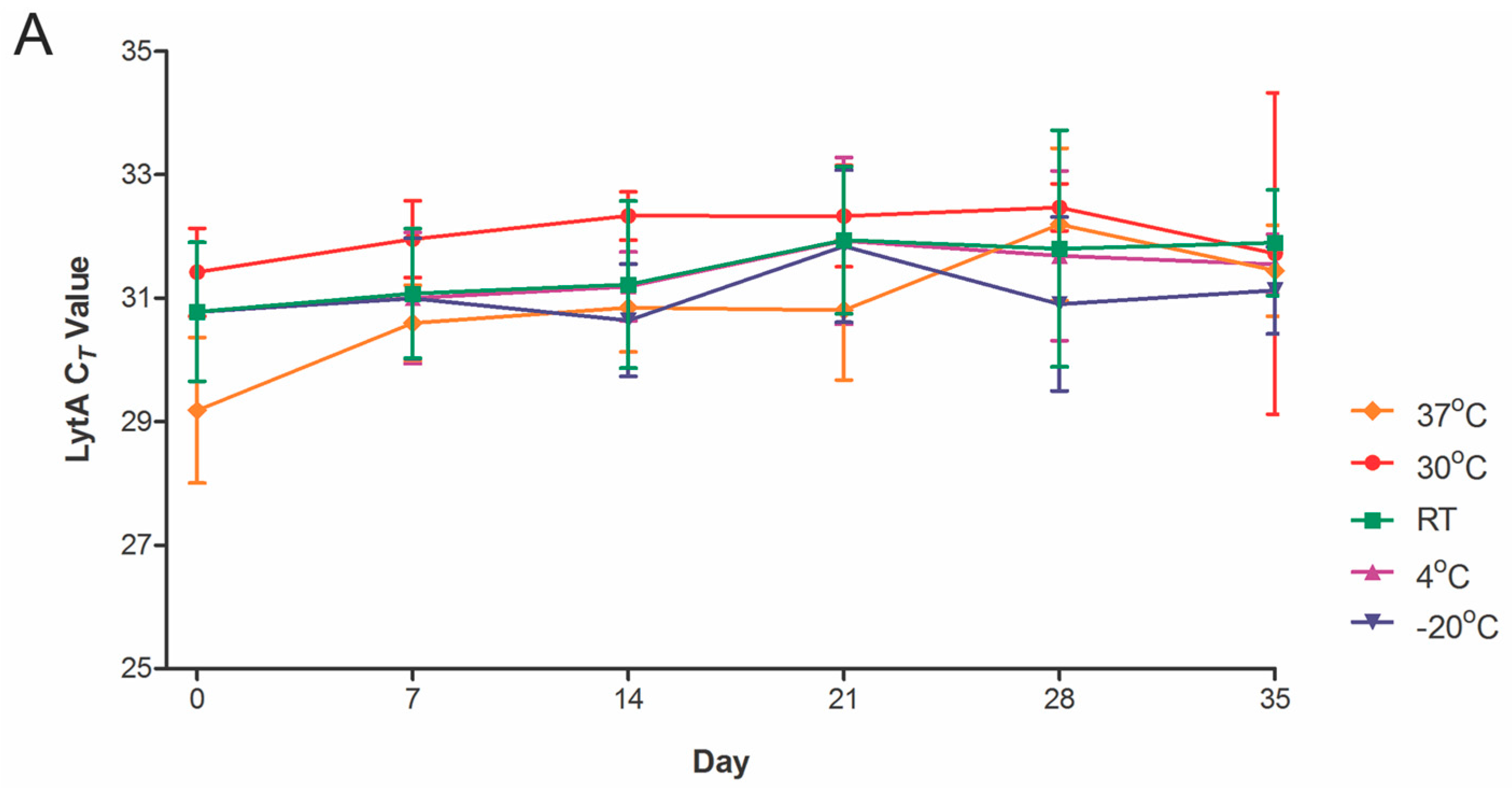
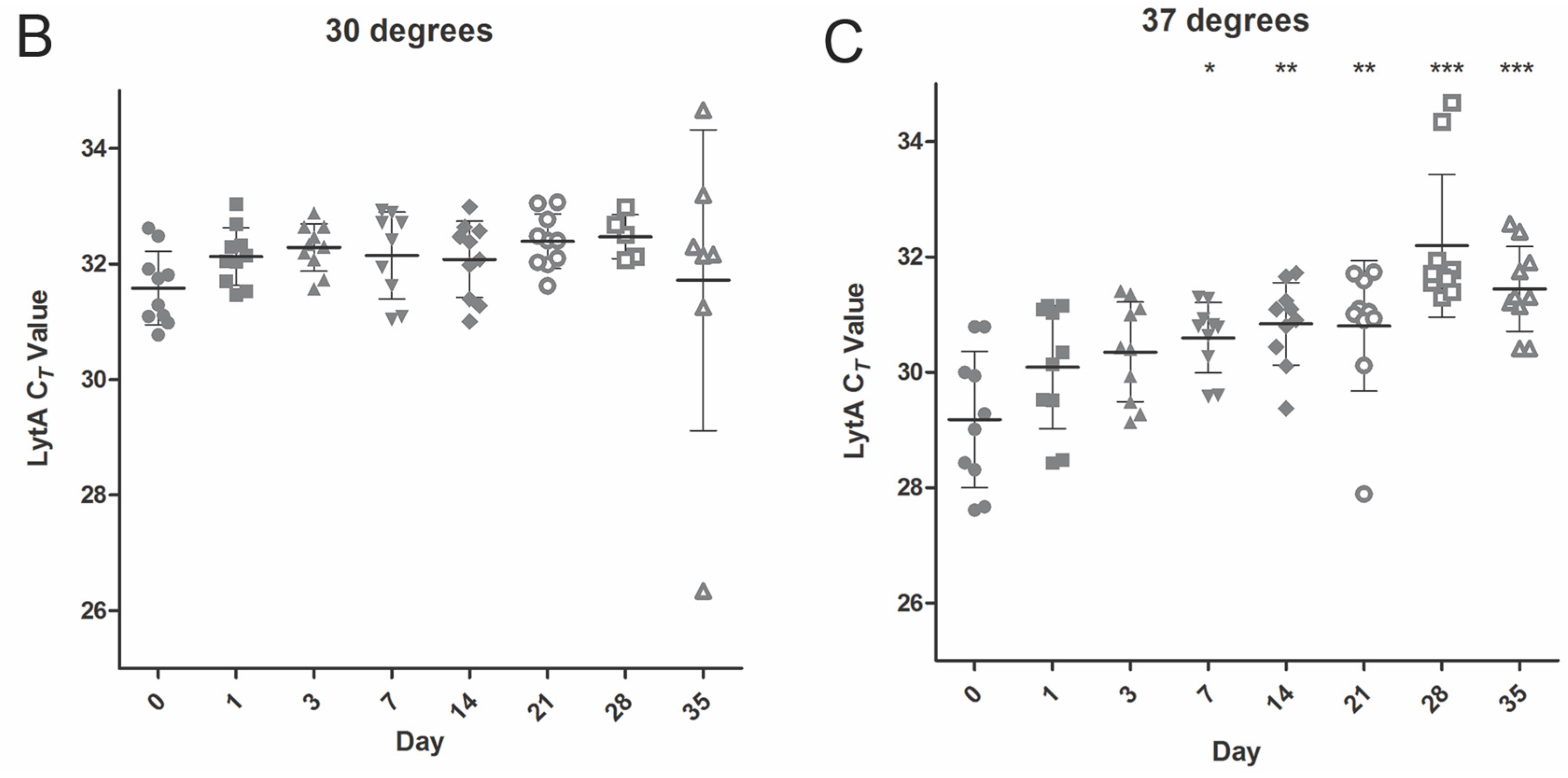
2.2. Temperature Stability
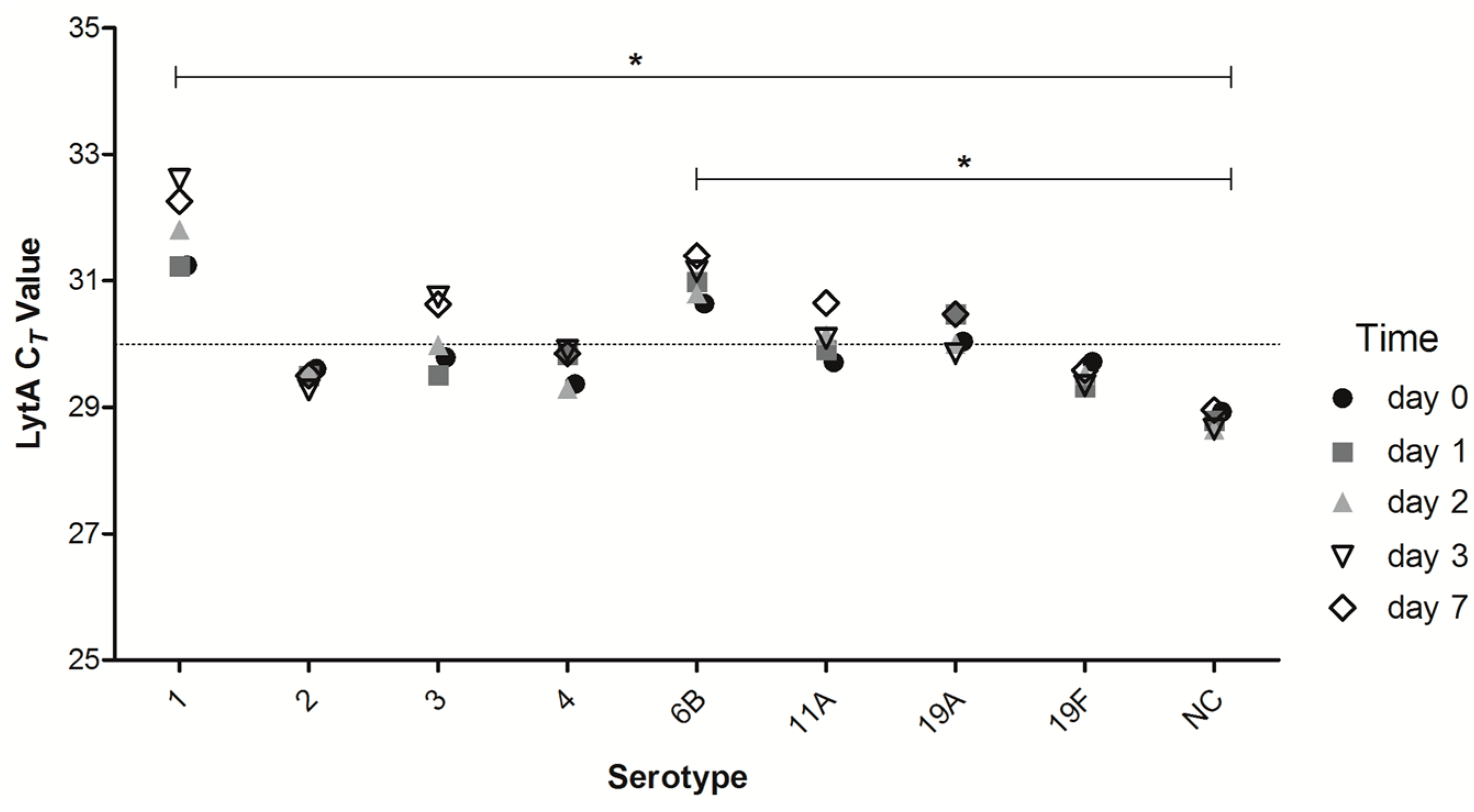
2.3. Short-Term DNA Stability of DSS of Different Strains of Streptococcus pneumoniae (S. pneumoniae)
2.4. Detection of Pneumococcal Presence in DSS Specimens from Clinical Saliva Samples
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Mock Saliva Experiments
4.3. Dried Saliva Spots
4.4. Clinical Study
4.5. Isolation of Bacterial DNA
4.6. Real-Time Quantitative PCR Targeting S. pneumoniae
4.7. Lower Limit of S. pneumoniae Detection
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DSS | dried saliva spot |
| qPCR | quantitative polymerase chain reaction |
| DNA | deoxyribonucleaic acid |
| CFU | colony forming unit |
| CT | cycle threshold |
| LOD | limit of detection |
References
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008, 6, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef]
- Bogaert, D.; de Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Dochez, A.R.; Avery, O.T. The occurrence of carriers of disease—Producing types of pneumococcus. J. Exp. Med. 1915, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, E.J.; Veenhoven, R.H.; Hak, E.; Rodenburg, G.D.; Bogaert, D.; Ijzerman, E.P.; Bruin, J.P.; van Alphen, L.; Sanders, E.A. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 2009, 302, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Spijkerman, J.; Prevaes, S.M.; van Gils, E.J.; Veenhoven, R.H.; Bruin, J.P.; Bogaert, D.; Wijmenga-Monsuur, A.J.; van den Dobbelsteen, G.P.; Sanders, E.A. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS ONE 2012, 7, e39730. [Google Scholar] [CrossRef]
- Van den Bergh, M.R.; Spijkerman, J.; Swinnen, K.M.; Francois, N.A.; Pascal, T.G.; Borys, D.; Schuerman, L.; Ijzerman, E.P.; Bruin, J.P.; van der Ende, A.; et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: A randomized controlled trial. Clin. Infect. Dis. 2013, 56, e30–e39. [Google Scholar] [CrossRef] [PubMed]
- Da Gloria Carvalho, M.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Heffron, R. Pneumonia: With Special Reference to Pneumococcus Lobar Pneumonia; Harvard Univeristy Press: Cambridge, MA, USA, 1939. [Google Scholar]
- White, B. The Biology of Pneumococcus; The Commonwealth Fund: New York, NY, USA, 1938. [Google Scholar]
- Gundel, M.; Okura, G. Untersuchungen über das gleichzeitige Vorkommen mehrer Pneumokokkentypen bei Gesunden und ihre Bedeutung für die Epidemiologie. Zeitschrift für Hyg. und Infekt. 1933, 114, 678–704. [Google Scholar] [CrossRef]
- Trzcinski, K.; Bogaert, D.; Wyllie, A.; Chu, M.L.; van der Ende, A.; Bruin, J.P.; van den Dobbelsteen, G.; Veenhoven, R.H.; Sanders, E.A. Superiority of trans-oral over trans-nasal sampling in detecting Streptococcus pneumoniae colonization in adults. PLoS ONE 2013, 8, e60520. [Google Scholar]
- Azzari, C.; Moriondo, M.; Indolfi, G.; Cortimiglia, M.; Canessa, C.; Becciolini, L.; Lippi, F.; de Martino, M.; Resti, M. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS ONE 2010, 5, e9282. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Chu, M.L.; Schellens, M.H.; van Engelsdorp Gastelaars, J.; Jansen, M.D.; van der Ende, A.; Bogaert, D.; Sanders, E.A.; Trzciński, K. Streptococcus pneumoniae in saliva of Dutch primary school children. PLoS ONE 2014, 9, e102045. [Google Scholar] [CrossRef] [PubMed]
- Krone, C.L.; Wyllie, A.L.; van Beek, J.; Rots, N.Y.; Oja, A.E.; Chu, M.L.; Bruin, J.P.; Bogaert, D.; Sanders, E.A.; Trzciński, K. Carriage of Streptococcus pneumoniae in aged adults with influenza-like-illness. PLoS ONE 2015, 10, e0119875. [Google Scholar] [CrossRef] [PubMed]
- Chiappin, S.; Antonelli, G.; Gatti, R.; de Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Nurkka, A.; Obiero, J.; Kayhty, H.; Scott, J.A. Effects of sample collection and storage methods on antipneumococcal immunoglobulin A in saliva. Clin. Diagn. Lab. Immunol. 2003, 10, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Nissen, F. Ein Vergleich des sog. Sputumsepticamiecoccus mit dem A. Frankel’schen Pneumonieerreger. Fortschr. Med. 1891, 9, 661. [Google Scholar]
- Snijdewind, I.J.; van Kampen, J.J.; Fraaij, P.L.; van der Ende, M.E.; Osterhaus, A.D.; Gruters, R.A. Current and future applications of dried blood spots in viral disease management. Antivir. Res. 2012, 93, 309–321. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W.; Williams, S.; Snodgrass, J.J. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 2007, 44, 899–925. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.V.; Alexander, J.R.; Adam, B.W.; Hannon, W.H. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 2001, 131, 1631S–1636S. [Google Scholar] [PubMed]
- Bertagnolio, S.; Penazzato, M.; Jordan, M.R.; Persaud, D.; Mofenson, L.M.; Bennett, D.E. World Health Organization generic protocol to assess drug-resistant HIV among children <18 months of age and newly diagnosed with HIV in resource-limited countries. Clin. Infect. Dis. 2012, 54, S254–S260. [Google Scholar] [PubMed]
- Peltola, H.; Roine, I.; Leinonen, M.; Kuisma, L.; Mata, A.G.; Arbo, A.; Goyo, J.; Saukkoriipi, A. Diagnosis of Streptococcus pneumoniae and Haemophilus influenzae type b meningitis by identifying DNA from cerebrospinal fluid-impregnated filter paper strips. Pediatr. Infect. Dis. J. 2010, 29, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Masciotra, S.; Khamadi, S.; Bile, E.; Puren, A.; Fonjungo, P.; Nguyen, S.; Girma, M.; Downing, R.; Ramos, A.; Subbarao, S.; et al. Evaluation of blood collection filter papers for HIV-1 DNA PCR. J. Clin. Virol. 2012, 55, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Denniff, P.; Spooner, N. Effect of storage conditions on the weight and appearance of dried blood spot samples on various cellulose-based substrates. Bioanalysis 2010, 2, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.L.; Camilli, A. Streptococcus pneumoniae is desiccation tolerant and infectious upon rehydration. MBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Trzcinski, K.; Lu, Y.J.; Bogaert, D.; Brandes, A.; Galagan, J.; Anderson, P.W.; Malley, R.; Lipsitch, M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009, 5, e1000476. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Trzcinski, K.; Meri, S.; Kayhty, H.; Vakevainen, M. The capsular serotype of Streptococcus pneumoniae is more important than the genetic background for resistance to complement. Infect. Immun. 2010, 78, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Nelson, K.E.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Peterson, S.; Heidelberg, J.; DeBoy, R.T.; Haft, D.H.; Dodson, R.J.; et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 2001, 293, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Malley, R.; Lipsitch, M.; Stack, A.; Saladino, R.; Fleisher, G.; Pelton, S.; Thompson, C.; Briles, D.; Anderson, P. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 2001, 69, 4870–4873. [Google Scholar] [CrossRef] [PubMed]
- Trzcinski, K.; MacNeil, A.; Klugman, K.P.; Lipsitch, M. Capsule homology does not increase the frequency of transformation of linked penicillin binding proteins PBP 1a and PBP 2x in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2005, 49, 1591–1592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Enright, M.C.; Spratt, B.G. Identification of the major Spanish clones of penicillin-resistant pneumococci via the internet using multilocus sequence typing. J. Clin. Microbiol. 2000, 38, 977–986. [Google Scholar] [PubMed]
- Elliott, I.; Dittrich, S.; Paris, D.; Sengduanphachanh, A.; Phoumin, P.; Newton, P.N. The use of dried cerebrospinal fluid filter paper spots as a substrate for PCR diagnosis of the aetiology of bacterial meningitis in the Lao PDR. Clin. Microbiol. Infect. 2013, 19, E466–E472. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, A.; Abdel-Rehim, M. Dried saliva spot as a sampling technique for saliva samples. Biomed. Chromatogr. 2014, 28, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Numako, M.; Takayama, T.; Noge, I.; Kitagawa, Y.; Todoroki, K.; Mizuno, H.; Min, J.Z.; Toyo’oka, T. Dried saliva spot (DSS) as a convenient and reliable sampling for bioanalysis: An application for the diagnosis of diabetes Mellitus. Anal. Chem. 2016, 88, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Mda, G.; Tondella, M.L.; McCaustland, K.; Weidlich, L.; McGee, L.; Mayer, L.W.; Steigerwalt, A.; Whaley, M.; Facklam, R.R.; Fields, B.; et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 2007, 45, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Albrich, W.C.; Madhi, S.A.; Adrian, P.V.; van Niekerk, N.; Mareletsi, T.; Cutland, C.; Wong, M.; Khoosal, M.; Karstaedt, A.; Zhao, P.; et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin. Infect. Dis. 2011, 54, 601–609. [Google Scholar] [CrossRef] [PubMed]

| Strain | Serotype | Description | Reference or Source |
|---|---|---|---|
| GA07694 | 1 | Invasive clinical isolate | [28] |
| GA03901 | 2 | Invasive clinical isolate | [29] |
| GA07650 | 3 | Invasive clinical isolate | [29] |
| TIGR4 | 4 | Invasive clinical isolate | [30] |
| 603 | 6B | Invasive clinical isolate | [31] |
| 603J | NT 1 | Acapsular variant of 603 | [28] |
| 1101 | 11A | Invasive clinical isolate | [32] |
| SJD86 | 19A | Invasive clinical isolate | [33] |
| ATCC6319 | 19F | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krone, C.L.; Oja, A.E.; Van de Groep, K.; Sanders, E.A.M.; Bogaert, D.; Trzciński, K. Dried Saliva Spots: A Robust Method for Detecting Streptococcus pneumoniae Carriage by PCR. Int. J. Mol. Sci. 2016, 17, 343. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030343
Krone CL, Oja AE, Van de Groep K, Sanders EAM, Bogaert D, Trzciński K. Dried Saliva Spots: A Robust Method for Detecting Streptococcus pneumoniae Carriage by PCR. International Journal of Molecular Sciences. 2016; 17(3):343. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030343
Chicago/Turabian StyleKrone, Cassandra L., Anna E. Oja, Kirsten Van de Groep, Elisabeth A. M. Sanders, Debby Bogaert, and Krzysztof Trzciński. 2016. "Dried Saliva Spots: A Robust Method for Detecting Streptococcus pneumoniae Carriage by PCR" International Journal of Molecular Sciences 17, no. 3: 343. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030343






