Biosurfactants: Multifunctional Biomolecules of the 21st Century
Abstract
:1. Introduction
2. Producing Microorganisms
3. Classification
3.1. Glycolipids
3.2. Fatty Acids, Phospholipids and Neutral Lipids
3.3. Polymeric Biosurfactants
3.4. Particulate Biosurfactants
4. Properties
4.1. Surface and Interfacial Activity
4.2. Tolerance to Temperature, pH and Ionic Strength
4.3. Biodegradability
4.4. Low Toxicity
4.5. Specificity
4.6. Biocompatibility and Digestibility
4.7. Emulsion Forming/Breaking
5. Factors Affecting Biosurfactant Production
5.1. Carbon Source
5.2. Nitrogen Sources
5.3. Growth Conditions
6. Metabolic Pathways of Biosurfactant Production
7. Physiology
8. Fermentation Kinetics
9. Raw Materials for Biosurfactant Production
9.1. Olive Oil Mill Effluent (OOME)
9.2. Animal Fat
9.3. Frying Oils
9.4. Soapstocks
9.5. Molasses
9.6. Whey
9.7. Corn Steep Liquor
9.8. Starchy Substrates
10. Recovery of Biosurfactants
11. Industrial Applications of Biosurfactants
11.1. Petroleum Recovery
11.2. Bioremediation
11.3. Removal of Hydrophobic Organic Pollutants
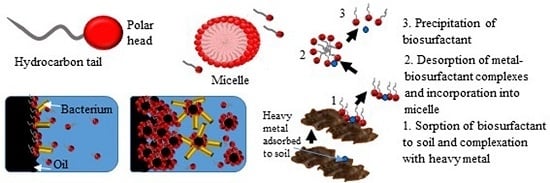
11.4. Removal of Heavy Metals
11.5. Food Industry
11.6. Medicine
11.7. Nanaotechnology
12. Future Directions and Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.F.S.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A.; Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar]
- Cerqueira, V.S.; Hollenbach, E.B.; Maboni, F.; Vainstein, M.H.; Camargo, F.A.; do Carmo, M.; Peralba, R.; Bento, F.M. Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour. Technol. 2011, 102, 11003–11010. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.F.C.; Freire, D.M.G.; Ferraz, E.C. Biosurfactant microfoam: Application in the removal of pollutants from soil. J. Environ. Chem. Eng. 2015, 3, 89–94. [Google Scholar] [CrossRef]
- Pacwa-Plociniczak, M.; Plaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 13, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. Appl. Microbiol. Biotechnol. 2011, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Paquot, M. From renewable vegetables resources to microorganisms: new trends in surfactants. C. R. Chimie 2004, 7, 641–646. [Google Scholar]
- Marchant, R.; Funston, S.; Uzoigwe, C.; Rahman, P.K.S.M.; Banat, I.M. Production of biosurfactants from nonpathogenic bacteria. In Biosurfactants; Taylor & Francis: New York, USA, 2014; pp. 73–81. [Google Scholar]
- Chakraborty, J.; Das, S. Biosurfactant-based bioremeditaion of toxic metals. In Microbial Biodegradation and Bioremediation, 1st ed.; Das, S., Ed.; Elsevier: Rourkela Odisha, India, 2014; pp. 167–201. [Google Scholar]
- Shepherd, R.; Rockey, J.; Shutherland, I.W.; Roller, S. Novel bioemulsifiers from microorganisms for use in foods. J. Biotechnol. 1995, 40, 207–217. [Google Scholar] [CrossRef]
- Bognolo, G. Biossurfactants as emulsifying agents for hydrocarbons. Coll. Surf. A Physicochem. Eng. Asp. 1999, 152, 41–52. [Google Scholar] [CrossRef]
- Pattanath, K.M.; Rahman, K.S.; Gakpe, E. Production, characterization and applications of biosurfactants—Review. Biotechnology 2008, 7, 360–370. [Google Scholar]
- Chrzanowski, L.; Ławniczak, L.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Deziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Hitsatsuka, K.; Nakahara, T.; Sano, N.; Yamada, K. Formation of a rhamnolipid by Pseudomonas aeruginosa and its function in hydrocarbon fermentation. Agric. Biol. Chem. 1971, 35, 686–692. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process Biochem. 2011, 46, 621–630. [Google Scholar] [CrossRef]
- Ławniczak, L.; Marecik, R.; Chrzanowski, L. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, L.; Dziadas, M.; Ławniczak, L.; Cyplik, P.; Białas, W.; Szulc, A.; Lisiecki, P.; Jelen, H. Biodegradation of rhamnolipids in liquid cultures: Effect of biosurfactant dissipation on diesel fuel/B20 blend biodegradation efficiency and bacterial community composition. Bioresour. Technol. 2012, 111, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Szulc, A.; Ambrozewicz, D.; Sydow, M.; Ławniczak, L.; Piotrowska-Cyplik, A.; Marecik, R.; Chrzanowski, L. The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: Feasibility during field studies. J. Environ. Manag. 2014, 132, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Marecik, M.; Wojtera-Kwiczor, J.; Ławniczak, L.; Cyplik, P.; Szulc, A.; Piotrowska-Cyplik, A.; Chrzanowski, L. Rhamnolipids increase the phytotoxicity of diesel oil towards four common plant species in a terrestrial environment. Water Air Soil Pollut. 2012, 223, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, L.; Wick, L.Y.; Meulenkamp, R.; Kaestner, M.; Heipieper, H.J. Rhamnolipid biosurfactants decrease the toxicity of chlorinated phenols to Pseudomonas putida DOT-T1E. Lett. Appl. Microbiol. 2009, 48, 756–762. [Google Scholar] [PubMed]
- Chrzanowski, L.; Owsianiak, M.; Szulc, A.; Marecik, R.; Piotrowska-Cyplik, A.; Olejnik-Schmidt, A.K.; Staniewski, J.; Lisiecki, P.; Ciesielczyk, F.; Jesionowski, T.; et al. Interactions between rhamnolipid biosurfactants and toxic chlorinated phenols enhance biodegradation of a model hydrocarbon-rich effluent. Int. Biodeterior. Biodegrad. 2011, 65, 605–611. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.J.; Sánchez, H.H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Pakshirajan, K. Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl. Biochem. Biotechnol. 2009, 158, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.K.; Tyagi, V.K. Microbial Surfactants: A review. J. Oleo Sci. 2006, 55, 155–166. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, L.K. Purification of lactonic sophorolipids by crystallization. J. Biotechnol. 2001, 87, 263–272. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.D.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lang, S. Biological amphiphiles (microbial biosurfactants). Curr. Opin. Coll. Interface Sci. 2002, 7, 12–20. [Google Scholar] [CrossRef]
- Hatha, A.A.M.; Edward, G.; Rahman, K.S.M.P. Microbial biosurfactants-review. J. Mar. Atmos. Res. 2007, 3, 1–17. [Google Scholar]
- Chakrabarti, S. Bacterial Biosurfactant: Characterization, Antimicrobial and Metal Remediation Properties. Ph.D. Thesis, National Institute of Technology, Surat, India, March 2012. [Google Scholar]
- Barros, F.F.C.; Quadros, C.P.; Maróstica, M.R.; Pastore, G.M. Surfactina: Propriedades químicas, tecnológicas e funcionais para aplicações em alimentos. Quím. Nova 2007, 30, 1–14. [Google Scholar] [CrossRef]
- Velikonja, J.; Kosaric, N. Biosurfactants in food applications. In Biosurfactants: Production, Properties, Applications; Kosaric, N., Sukan, F.V., Eds.; CRC Press: New York, NY, USA, 1993; pp. 419–448. [Google Scholar]
- Cirigliano, M.C.; Carman, G.M. Purification and characterization of liposan, a bioemulsifier from Candida lipolytica. Appl. Microbiol. Biotechnol. 1985, 50, 846–850. [Google Scholar]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [PubMed]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactants from Fungi: A Review. Pet. Environ. Biotechnol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Cooper, D.G.; Paddock, D.A. Production of a biosurfactant from Torulopsis bombicola. Appl. Environ. Microbiol. 1984, 47, 173–176. [Google Scholar] [PubMed]
- Kim, S.Y.; Oh, D.K.; Lee, K.H.; Kim, J.H. Effect of soybean oil and glucose on sophorose lipid fermentation by Torulopsis bombicola in continuous culture. Appl. Microbiol. Biotechnol. 1997, 48, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.A.; de Lara, S.G.; Garcia-Ochoa, F. Optimization of a synthetic medium for Candida bombicola growth using factorial design of experiments. Enzyme Microb. Technol. 1997, 21, 221–229. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2007, 54, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microbiol. Biotechnol. 2007, 23, 729–734. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Porto, A.L.F.; Campos-Takaki, G.M. Bioemulsifier production in batch culture using glucose as carbon source by Candida lipolytica. Appl. Biochem. Biotechnol. 2001, 95, 59–67. [Google Scholar] [CrossRef]
- Bednarski, W.; Adamczak, M.; Tomasik, J.; Plaszvzyk, M. Application of oil refinery waste in the biosynthesis of glycolipids by yeast. Bioresour. Technol. 2004, 95, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Ikegami, T.; Suzuki, G.T.; Sasaki, A.; Takeyama, Y.; Idemoto, Y.; Koura, N.; Yanagishita, H. Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids by Pseudozyma (Candida antartica). Biotechnol. Lett. 2001, 23, 1709–1714. [Google Scholar] [CrossRef]
- Amezcua-Veja, C.; Poggi-Varaldo, H.M.; Esparza-Garcia, F.; Rodriguez-Vazquez, R. Effect of culture conditions on fatty acids composition of a biosurfactant produced by Candida ingens and of surface tension of culture media. Bioresour. Technol. 2006, 98, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Mercadé, M.E.; Bosch, M.P.; Parra, J.L.; Espiny, M.J.; Manresa, M.A.; Guinea, J. Effect of the carbon source on biosurfactant production by Pseudomonas aeruginosa 44T1. Biotechnol. Lett. 1989, 11, 871–874. [Google Scholar] [CrossRef]
- Santa Anna, I.M.; Sebastian, G.V.; Pereira, N., Jr.; Alves, T.L.M.; Menezes, E.P.; Freire, D.M.G. Production of biosurfactant from a new and promissing strain of Pseudomonas aeruginosa PA1. Appl. Biochem. Biotechnol. 2002, 91, 459–467. [Google Scholar]
- Mulligan, C.N.; Gibbs, B.F. Correlation of nitrogen metabolism with biosurfactant production by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1989, 55, 3016–3019. [Google Scholar] [PubMed]
- Deshpande, M.; Daniels, L. Evaluation of sophorolipid biosurfactant production by Candida bombicola using animal fat. Bioresour. Technol. 1995, 54, 143–150. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, B.D.; Choung, D.H.; Oh, H.M.; Katsuragi, T. Characterization of a biosurfactant mannosylerythritol lipid produced from Candida sp. SY 16. Appl. Microbiol. Biotechnol. 1999, 52, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Sarubbo, L.A.; Benicio, B.N.; Campos-Takaki, G.M. Experimental design for the production of tensio-active agent by Candida lipolytica. J. Ind. Microbiol. Biotechnol. 2008, 35, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Luna, J.M.; Campos-Takaki, G.M. Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electr. J. Biotechnol. 2006, 9, 400–406. [Google Scholar]
- Cirigliano, M.C.; Carman, G.M. Isolation of a bioemulsifier from Candida lipolytica. Appl. Environ. Microbiol. 1984, 48, 747–750. [Google Scholar] [PubMed]
- Konishi, M.; Fukuoka, T.; Morita, T.; Imura, T.; Kitamoto, D. Production of new types of sophorolipids by Candida batistae. J. Oleo Sci. 2008, 57, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Coll. Surf. B Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Thaniyavarn, J.; Chianguthai, T.; Sangvanich, P.; Roongsawang, N.; Washio, K.; Morikawa, M.; Thaniyavarn, S. Production of sophorolipid biosurfactant by Pichia anomala. Biosci. Biotechnol. Biochem. 2008, 72, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Cavalero, D.A.; Cooper, D.G. The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J. Biotechnol. 2003, 103, 31–41. [Google Scholar] [CrossRef]
- Felse, P.A.; Shah, V.; Chan, J.; Rao, K.J.; Gross, R.A. Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme Microb. Technol. 2007, 40, 316–323. [Google Scholar] [CrossRef]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Coll. Surf. B Biointerfaces 2010, 79, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.J.S.; Vazquez, L.; de Campos, N.P.; de Franca, F.P. Production of rhamnolipids by a Pseudomonas alcaligenes strain. Process Biochem. 2009, 44, 383–389. [Google Scholar] [CrossRef]
- Cunha, C.D.; Rosario, M.; Rosado, A.S.; Leite, G.F. Serratia sp. SVGG16: A promising biosurfactant producer isolated from tropical soil during growth with ethanol-blended gasoline. Process Biochem. 2004, 39, 2277–2282. [Google Scholar] [CrossRef]
- Weber, L.; Doge, C.; Haufe, G.; Hommel, R.; Kleber, H.-P. Oxygenation of hexadecane in the biosynthesis of cyclic glycolipids in Torulopsis apicola. Biocatal 1992, 5, 267–292. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hommel, R.K.; Huse, K. Regulation of sophorose lipid production by Candida (Torulopsis) apicola. Biotechnol. Lett. 1993, 15, 853–858. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Nomura, N.; Uchiyama, H.; Imura, T.; Morita, T.; Fukuoka, T.; Kitamoto, D. Structural characterization and surface-active properties of a succinoyl trehalose lipid produce by Rhodococcus sp. SD-74. J. Oleo Sci. 2009, 58, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Syldatk, C.; Wagner, F. Production of biosurfactants. In Biosurfactants and Biotechnology; Kosaric, N., Cairns, W.L., Gray, N.C.C., Eds.; Marcel Dekker: New York, NY, USA, 1987; pp. 89–120. [Google Scholar]
- Tan, H.M. Biosurfactants and their roles in bioremediation. Cheong Jit Kong 2000, 1–12. [Google Scholar]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S. An update on the use of uncoventional substrates for biosurfactant production and their new applications. Appl. Micobiol. Biotechnol. 2002, 58, 428–434. [Google Scholar]
- Batista, R.M.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L.A. Effect of Medium components on the production of a biosurfactant from Candida tropicalis applied to the removal of hydrophobic contaminants in soil. Water Environ. Res. 2010, 82, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Mercade, M.E.; Manresa, A.; Robert, M.; Espuny, M.J.; Deandres, C.; Guinea, J. Olive oil mill effluent (OOME). New substrat for biosurfactant production. Bioresour. Technol. 1993, 43, 1–6. [Google Scholar] [CrossRef]
- Fox, S.I.; Bala, G.A. Production of surfactant from Bacillus subtilis ATTCC 21332 using potato substrates. Bioresour. Technol. 2000, 75, 235–240. [Google Scholar] [CrossRef]
- Thompson, D.N.; Fox, S.L.; Bala, G.A. Biosurfactants from potato process effluents. Appl. Biochem. Biotechnol. 2000, 84, 917–930. [Google Scholar] [CrossRef]
- Maneerat, S. Production of biosurfactants using substrates from renewable-resources. Songklanakarin J. Sci. Technol. 2005, 27, 675–683. [Google Scholar]
- Santos, D.K.F.; Brandão, Y.B.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Santos, V.A.; Sarubbo, L.A. Optimization of cultural conditions for biosurfactant production from Candida lipolytica. Biocatal. Agric. Biotechnol. 2014, 3, 48–57. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Gusmão, C.A.B.; Rufino, R.D.; Sarubbo, L.A. Laboratory production and characterization of a new biosurfactant from Candida glabrata UCP 1002 cultivated in vegetable fat waste applied to the removal of hydrophobic contaminant. World J. Microbiol. Biotechnol. 2010, 26, 1683–1692. [Google Scholar] [CrossRef]
- Alcântara, R.; Amores, J.; Canoira, L.; Fidalgo, E.; Franco, M.J.; Navarro, A. Catalytic production of biodiesel from soy-hean oil, used frying oil and tallow. Biomass Bioenergy 2000, 18, 515–527. [Google Scholar] [CrossRef]
- Cvengros, Z.; Cvengrosova, Z. Used fruing oils and fats and their utilization in the production of methyl ester of higher fatty acids. Biomass Bioenergy 2004, 27, 173–181. [Google Scholar] [CrossRef]
- Haba, E.; Espuny, M.J.; Busquets, M.; Manresa, A. Screening and production of rhamnolipids Pseudomonas aeruginosa 47T2 NCIB 40044 from waste flying oils. J. Appl. Microbiol. 2000, 88, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Contiero, J.; Manresa, M.A.; Moraes, I.O. Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J. Food Eng. 2002, 54, 283–288. [Google Scholar] [CrossRef]
- Shabtai, Y. Production of exopolysaccharides by Acinetobacter strains in a controlled fed-batch fermentation process using soap stock oil (SSO) as carbon source. Int. J. Biol. Macromol. 1990, 12, 145–152. [Google Scholar] [CrossRef]
- Ghurye, G.L.; Vipulananda, C.; Wilson, R.C. A practical approach to biosurfactant production using nonaseptic fermantation of mixed cultures. Biotechnol. Bioeng. 1994, 44, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, S.; Iakovidou, G.; Liakopoulou-Kyriakides, M.; Kyriakidis, D.A.; Skaracis, G.N. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003, 39, 249–256. [Google Scholar] [CrossRef]
- Lazaridou, A.; Roukas, T.; Biliaderis, C.G.; Vaikousi, H. Characterization of pullulan produced from beet molasses by Aureobasidium pullulans in a stirred tank reactor under varying agitation. Enzyme Microbiol. Technol. 2002, 31, 122–132. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Utilization of molasses for biosurfactant production by two Bacillus strains at thermophilic conditions. J. Am. Oil Chem. Soc. 1997, 74, 887–889. [Google Scholar] [CrossRef]
- Sudhakar-Babu, P.; Vaidya, A.N.; Bal, A.S.; Kapur, R.; Juwarka, A.; Khanna, P. Kinetics of biosurfactants production by Pseudomonas aeruginosa strain from industrial wastes. Biotechnol. Lett. 1996, 18, 263–268. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Albuquerque, C.D.C.; Sarubbo, L.A.; Campos-Takaki, G.M. Economic optimized medium for tenso-active agent production by Candida sphaerica UCP 0995 and application in the removal of hydrophobic contaminant from sand. Int. J. Mol. Sci. 2011, 12, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Luna, J.M.; Rodrigues, G.I.B.; Campos-Takaki, G.M.; Sarubbo, L.A.; Ferreira, S.R.M. Application of a yeast biosurfactant in the removal of heavy metals and hydrophobic contaminant in a soil used as slurry barrier. Appl. Environ. Soil Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Campos-Takaki, G.M.; Leite, L.F.C.; Sarubbo, L.A. Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP 0995. Process Biochem. 2008, 43, 912–917. [Google Scholar] [CrossRef]
- Nitschke, M.; Ferraz, C.; Pastore, G.M. Selection of microorganisms for biosurfactant production using agroindustrial wastes. Braz. J. Microbiol. 2004, 435, 81–85. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP 0995 for application in the petroleum industry. Coll. Surf. B Biointerfaces 2013, 102, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Evaluation antimicrobial and antiadhesive properties of the biosurfactant Lunasan produced by Candida sphaerica UCP 0995. Curr. Microbiol. 2011, 62, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarubbo, L.A.; Marçal, M.C.; Neves, M.L.C.; Porto, A.L.F.; Campos-Takaki, G.M. The use of babassu oil as substrate to produce bioemulsifiers by Candida lipolytica. Can. J. Microbiol. 1999, 45, 1–4. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khan, M.S.; Khalid, Z.M.; Rehman, A. Production kinetics and tensioactive characteristics of biosurfactant from a Pseudomonas aeruginosa mutant grown on waste frying oils. Biotechnol. Lett. 2006, 28, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Rehman, A.; Khan, M.S.; Khalid, Z.M. Improved production of biosurfactant by a Pseudomonas aeruginosa mutant using vegetable oil refinery wastes. Biodegradation 2007, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2007, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.V.; Juwarkar, A.A. Distillery and curd whey wastes as viable alternative sources for biosurfactant production. World J. Microbiol. Biotechnol. 2001, 17, 61–69. [Google Scholar] [CrossRef]
- Dubey, K.V.; Juwarkar, A.A.; Singh, S.K. Adsorption-desorption process using woodbased activated carbon for recovery of biosurfactant from fermented distillery wastewater. Biotechnol. Prog. 2005, 21, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.R.; Teixeira, J.A.; Oliveira, R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 2006, 32, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Helmy, Q.; Kardena, E.; Funamizu, N.; Wisjnuprapto, W. Strategies toward commercial scale of biosurfactant production as potential substitute for it’s chemically counterparts. Int. J. Biotechnol. 2011, 12, 66–86. [Google Scholar]
- Akhtar, M.; Lentz, M.J.; Banchette, R.A.; Kirk, T.K. Corn steep liquor lowers the amount of inoculum for biopulping. TAPPI J. 1997, 80, 161–164. [Google Scholar]
- Cardinal, E.V.; Hedrick, L.R. Microbiological assay of corn steep liquor for amino acid content. J. Biol. Chem. 1948, 172, 609–612. [Google Scholar] [PubMed]
- Luna, J.M.; Rufino, R.D.; Jara, A.M.T.; Brasileiro, P.P.F.; Sarubbo, L.A. Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Coll. Surf. A Physicochem. Eng. Asp. 2015, 480, 413–418. [Google Scholar] [CrossRef]
- Silva, N.M.P.R.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatal. Agric. Biotechnol. 2014, 3, 132–139. [Google Scholar]
- Muthusamy, K.; Gopalakrishnan, S.; Ravi, T.K.; Sivachidambaram, P. Biosurfactants: Properties, commercial production and application. Curr. Sci. 2008, 94, 736–747. [Google Scholar]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Winterburn, J.B.; Russell, A.B.; Martin, P.J. Integrated recirculating foam fractionation for the continuous recovery of biosurfactant from fermenters. Biochem. Eng. J. 2011, 54, 132–139. [Google Scholar] [CrossRef]
- Rangarajan, V.; Sem, R. An inexpensive strategy for facilitated recovery of metals and fermentation products by foam fractionation process. Coll. Surf. B Biointerfaces 2013, 104, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.R.; Banat, I.M.; Teixeira, J.A.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobrinho, H.B.S.; Luna, J.M.; Rufino, R.D.; Porto, A.L.F.; Sarubbo, L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- US Energy Information Administration. Annual Energy Review, 2010. Available online: http://large.stanford.edu/courses/2012/ph241/druzgalski2/docs/aer.pdf (accessed on 05 September 2015).
- Summers, D. Enhancing Oil Recovery: A Look at Stripper Wells. Available online: http//www.oilprice.com (accessed on 10 January 2011).
- Austad, T.; Taugbøl, K. Chemical flooding of oil reservoirs 1. Low tension polymer flood using a polymer gradient in the three-phase region. Coll. Surf. A Phys. Eng. Asp. 1995, 101, 87–97. [Google Scholar] [CrossRef]
- West, C.C.; Harwell, J.H. Surfactant and subsurface remediation. Environ. Sci. Technol. 1992, 26, 2324–2330. [Google Scholar] [CrossRef]
- Sabatini, D.A.; Harwell, J.H.; Knox, R.C. Surfactant selection criteria for enhanced subsurface remediation. In Innovative Subsurface Remediation; Brusseau, M.L., Sabatini, D.A., Gierke, J.S., Annable, M.D., Eds.; American Chemical Society: Washington, WA, USA, 1999; pp. 8–23. [Google Scholar]
- Sabatini, D.A.; Knox, R.C.; Harwell, J.H.; Wu, B. Integrated design of surfactant enhanced DNAPL remediation: Efficient super solubilization and gradient systems. J. Contam. Hydrol. 2000, 45, 99–121. [Google Scholar] [CrossRef]
- Souza, E.C.; Vessoni-Penna, T.C.; Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Rocha, R.B., Jr.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Microbial derived surface active compounds: Properties and screening concept. World J. Microbiol. Biotechnol. 2015, 31, 1001–1020. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; De Rienzo, M.A.D.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N. Recent advances in the environmental applications of biosurfactants. Curr. Opin. Coll. Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA), U.S. Treatment Technologies for Site Cleanup: Annual Status Report, 10th ed.; Office of Solid Waste and Emergency Response: Cincinnati, Ohio, USA, 2001. [Google Scholar]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, M.; Mohebali, G.; Haddadi, A.; Shakarami, H.; Nuhi, A. Emulsification potential of a newly isolated biosurfactantproducing bacterium, Rhodococcus sp. strain TA6. Coll. Surf. B Biointerfaces 2011, 82, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kuyukina, M.S.; Ivshina, I.B.; Makarov, S.O.; Litvinenko, L.V.; Cunningham, C.J.; Philip, J.C. Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environ. Int. 2005, 31, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Aparna, A.; Srinikethan, G.; Hedge, S. Effect of addition of biosurfactant produced by Pseudomonas ssp. on biodegradation of crude oil. In International Proceedings of Chemical, Biological & Environmental Engineering, Proceedings of the 2nd International Proceedings of Chemical, Singapore, Singapore, 26–28 February 2011; Volume 6, pp. 71–75.
- Franzetti, A.; Caredda, P.; Ruggeri, C.; La Colla, P.; Tamburini, E.; Papacchini, M.; Bestetti, G. Potential applications of surface active compounds by Gordonia sp. strain BS29 in soil remediation technologies. Chemosphere 2009, 75, 810–807. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Brusseau, M.L.; Miller, R.M. Biosurfactant-enhanced removal of residual hydrocarbon from soil. J. Contam. Hydrol. 1997, 25, 157–170. [Google Scholar] [CrossRef]
- Silva, R.C.F.S.; Rufino, R.D.; Luna, J.M.; Farias, C.B.B.; Filho, H.J.B.; Santos, V.A.; Sarubbo, L.A. Enhancement of biosurfactant production from Pseudomonas cepacia CCT6659 through optimisation of nutritional parameters using Response Surface Methodology. Tenside Surf. Det. 2013, 50, 137–142. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Ferreira, I.N.S.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Marinho, P.H.C.; Farias, C.B.B.; Ferreira, S.R.M.; Sarubbo, L.A. Removal of petroleum derivative adsorbed to soil by biosurfactant Rufisan produced by Candida lipolytica. J. Pet. Sci. Eng. 2013, 109, 117–122. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Coll. Surf. B Biointerfaces 2011, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Wang, S. Remediation of a heavy metal contaminated soil by a rhamnolipid foam. In Geoenvironmental Engineering: Integrated Management of Groundwater and Contaminated Land; Thomas Telford: London, UK, 2004; pp. 544–551. [Google Scholar]
- Singh, A.; Van-Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.; Kundu, D.; Chaudhari, A. Biosurfactant-assisted bioaugmentation in bioremediation. In Microorganisms in Environmental Management: Microbes and Environment, 1st ed.; Satyanarayana, T., Johri, B.N., Prakash, A., Eds.; Springer: New York, NY, USA, 2012; pp. 631–664. [Google Scholar]
- Ochoa-Loza, F.J.; Artiola, J.F.; Maier, R.M. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J. Environ. Qual. 2001, 30, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, V.; Agrawal, N.; Tomar, R. Sorption of chromate and arsenate bysurfactant modified erionite (E-SMZ). J. Dispers. Sci. Technol. 2012, 33, 919–927. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Asçi, Y.; Nurbas, M.; Açikel, Y. A comparative study for the sorption of Cd(II) by soils with different clay contents and mineralogy and the recovery of Cd(II) using rhamnolipid biosurfactant. J. Hazard. Mater. 2008, 154, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.; Traver, R.P.; Downey, D.C. Surfactant enhanced in situ soil washing. US.EPA HWERL: Edilsin, NJ, USA, 1987. [Google Scholar]
- Herman, D.C.; Artiola, J.F.; Miller, R.M. Removal of cadmium, lead and zinc from soil by a rhamnolipid biosurfactant. Environ. Sci. Technol. 1995, 29, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Metal removal from contaminated soil and sediments by the biosurfactant surfactin. Environ. Sci. Technol. 1999, 33, 3812–3820. [Google Scholar] [CrossRef]
- Ochoa-Loza, F.J.; Noordman, W.H.; Jannsen, D.B.; Brusseau, M.L.; Maier, R.M. Effect of clays, metal oxides, and organic matter on rhaminolipid biosurfactant sorpition by soil. Chemosphere 2007, 66, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mukherjee, S.; Sen, R. Antiadhesive action of a marine microbial surfactant. Coll. Surf. B Biointerfaces 2009, 71, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Stacey, S.P.; Mclaughlin, M.J.; Kirby, J.K. Biodegradation of rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biol. Biochem. 2009, 41, 2214–2221. [Google Scholar] [CrossRef]
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functions and potential applications of glycolipid biosurfactants: From energy-saving materials to gene delivery carriers. J. Biosci. Bioeng. 2002, 94, 187–201. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Biossurfactantes: Propriedades e aplicações. Química Nova 2002, 25, 772–776. [Google Scholar] [CrossRef]
- Slizovskiy, I.B.; Kelsey, J.W.; Hatzinger, P.B. Surfactant-facilitated remediationof metal-contaminated soils: Efficacy and toxicological consequences to earth worms. Environ. Toxicol. Chem. 2011, 30, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.R.; Dias, A.C.; Mucha, A.P.; Bordalo, A.A.; Vasconcelos, M.T.S.D. Influence of surfactants on the Cu phytoremediation potential of a saltmarsh plant. Chemosphere 2009, 75, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Huang, Y.M.; Fan, C.-W.; Chen, C.-C.; Li, C.-Y.; Hsu, C.-M.; Chang, Y.-F.; Wu, C.-I.; Chen, C.-Y.; Jean, J.-S. Evaluation of remediation process with soapberry derived saponin for removal of heavy metals from contaminated soils in Hai-Pu Taiwan. J. Environ. Sci. 2013, 25, 1180–1185. [Google Scholar] [CrossRef]
- Ozturk, S.; Kaya, T.; Aslim, B.; Tan, S. Removal and reduction of chromium by Pseudomonas spp. and their correlation to rhamnolipid production. J. Hazard. Mater. 2012, 231, 64–69. [Google Scholar] [PubMed]
- Albuquerque, C.F.; Luna-Finkler, C.L.; Rufino, R.D.; Luna, J.M.; Menezes, C.T.B.; Santos, V.A.; Sarubbo, L.A. Evaluation of biosurfactants for removal of heavy metal ions from aqueous effluent using flotation techniques. Int. Rev. Chem. Eng. 2012, 4, 156–161. [Google Scholar]
- Menezes, C.T.B.; Barros, E.C.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage using the dissolved air flotation technique. Appl. Biochem. Biotechnol. 2011, 163, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Production of a Bioemulsifier with Potential Application in the Food Industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.M.; Stamford, T.L.M.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicol. Rep. 2015, 2, 1164–1170. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Shojaosadati, S.A.; Tehrani, H.A. Preparation and characterization of bioemulsifier from Saccharomyces cerevisiae. Lebensm. Wiss. Technol. 1996, 29, 734–737. [Google Scholar] [CrossRef]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; García, F.; Manresa, A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant sophorolipid acts in synergy with antibiotics to enhance their efficiency. BioMed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential therapeutic applications of microbial surface-active compounds. Bioengineering 2015, 2, 144–162. [Google Scholar]
- Robbel, L.; Marahiel, M.A. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J. Biol. Chem. 2010, 285, 27501–27508. [Google Scholar] [CrossRef] [PubMed]
- Tally, F.P.; Zeckel, M.; Wasilewski, M.M. Daptomycin, A novel agent for Gram-positive infections. Expert Opin. Investig. Drug 1999, 8, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Zottola, E.A. Microbial attachment and biofilm formation: A new problem for the food industry? Food Technol. 1994, 48, 107–114. [Google Scholar]
- Meylheuc, T.; Van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactants on solid surfaces and consequences regarding the bioahesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Morita, T.; Fukuoka, T.; Konishi, M.; Imura, T. Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr. Opin. Coll. Interface Sci. 2009, 14, 315–328. [Google Scholar] [CrossRef]
- Kiran, G.S.; Sabu, A.; Selvin, J. Synthesis of silver nanoparticles by glycolipid biosurfactant produced from marine Brevibacterium casei MSA19. J. Biotechnol. 2010, 148, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, P. Biosurfactant mediated synthesis of NiO nanorods. Mater. Lett. 2008, 62, 743–746. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.-Y.; Baker, S.C.; Chen, C.-C.; Jean, J.-S.; Fan, C.-W.; Chen, H.-R.; Wang, J.-C. Synthesis of silver nanoparticles using surfactin: A biosurfactant stabilizing agent. Mater Lett. 2009, 63, 1227–1230. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Silva, A.F.; Rufino, R.D.; Luna, J.M.; Souza, J.E.; Sarubbo, L.A. Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef]
- Biswas, M.; Raichur, A.M. Electrokinetic and rheological properties of nano zirconia in the presence of rhamnolipid biosurfactant. J. Am. Ceram. Soc. 2008, 91, 3197–3201. [Google Scholar] [CrossRef]
- Santos, H.F.; Carmo, F.L.; Paes, J.E.S.; Rosado, A.S.; Peixoto, R.S. Bioremediation of mangroves impacted by petroleum. Water Air Soil Pollut. 2011, 216, 329–350. [Google Scholar] [CrossRef]









| Biosurfactant Class | |||||
|---|---|---|---|---|---|
| Glycolipids | Polymeric Surfactants | Lipopeptides | Fatty Acids | Particulate Surfactant | Phospholipids |
| Producer microorganisms | |||||
| Acinetobacter calcoaceticus | Acinetobacter calcoaceticus Acinetobacter calcoaceticus Acinetobacter calcoaceticus Acinetobacter calcoaceticus Bacillus stearothermophilus Candida lipolytica Candida utilis Halomonas eurihalina Mycobacterium thermoautotrophium Sphingomonas paucimobilis | Acinetobacter sp. Bacillus licheniformis Bacillus pumilus Bacillus subtilis Candida lipolytica Gluconobacter cerinus Pseudomonas fluorescens Serratia marcescens Streptomyces sioyaensis Thiobacillus thiooxidans | Arthrobacter paraffineus Capnocytophaga sp. Corynebacterium insidibasseosum Corynebacterium lepus Nocardia erythropolis Penicillium spiculisporum Talaramyces trachyspermus | Acinetobacter calcoaceticus Cyanobacteria Pseudomonas marginalis | Acinetobacter sp. Aspergillus Corynebacterium lepus |
| Alcanivorax borkumensis | |||||
| Arthrobacter paraffineus | |||||
| Arthrobacter sp. | |||||
| Candida antartica | |||||
| Candida apicola | |||||
| Candida batistae | |||||
| Candida bogoriensis | |||||
| Candida bombicola | |||||
| Candida ishiwadae | |||||
| Candida lipolytica | |||||
| Lactobacillus fermentum | |||||
| Nocardia sp. | |||||
| Pseudomonas aeruginosa | |||||
| Pseudomonas sp. | |||||
| Rhodococcus erythropolis | |||||
| Rhodotorula glutinus | |||||
| Rhodotorula graminus | |||||
| Serratia marcescens | |||||
| Tsukamurella sp. | |||||
| Ustilago maydis | |||||
| Process | Biosurfactant Type | Biosurfactant Property Responsible for Separation | Advantages | |
|---|---|---|---|---|
| Batch mode | Acid precipitation | Surfactin | Biosurfactants become insoluble at low pH values | Low cost, efficient in crude biosurfactant recovery |
| Organic solvent extraction | Trehalolipids; Sophorolipids; Liposan | Biosurfactants are soluble in organic solvents due to the hydrophobic end | Efficient in crude biosurfactant recovery and partial purification, reusable nature | |
| Ammonium sulphate precipitation | Emulsan; Biodispersan; Lipopeptides | Salting-out of polymeric or protein-rich biosurfactants | Effective in isolation of certain type of polymeric biosurfactants | |
| Continuous mode | Adsorption to wood-activated carbon | Rhamnolipids; Lipopeptides; Glycolipids; Mannosylerythritol Lipids (MEL) | Biosurfactants are adsorbed to activated carbon and can be desorbed using organic solvents | Highly pure biosurfactants, cheaper, reusability, recovery from continuous culture |
| Adsorption to polystyrene resines | Rhamnolipids; Lipopeptides; Glycolipids; MEL | Biosurfactants are adsorbed to polystyrene resins and subsequently desorbed using organic solvents | Highly pure biosurfactants, cheaper, reusability, recovery from continuous culture | |
| Centrifugation | Glycolipids | Insoluble biosurfactants are precipitated due to centrifugal force | Reusable, effective in crude biosurfactant recovery | |
| Ion-exchange chromatography | Glycolipids | Charged biosurfactants are attached to ion-exchange resins and can be eluted with buffer | High purity, reusability, fast recovery | |
| Foam fractionation | Surfactin | Biosurfactant form and partition into foam | Useful in continuous recovery processes, high purity of product | |
| Ultrafiltration | Glycolipids | Biosurfactants form micelles above their critical micelle concentration (CMC), which are trapped by polymeric membranes | Fast, one-step recovery, high level of purity, reusability | |
| Industry | Application | Role of Biosurfactants | References |
|---|---|---|---|
| Environment | Bioremediation; Oil spill cleanup operations; Soil remediation and flushing | Emulsification of oils, lowering of interfacial tension, dispersion of oils, solubilisation of oils, wetting, spreading, detergency, foaming, corrosion inhibition in fuel oils and equipment, soil flushing. | [2,8] |
| Petroleum | Enhanced oil recovery; De-emulsification | Emulsification of oils, lowering of interfacial tension, de-emulsification of oil emulsions, solubilisation of oils, viscosity reduction, dispersion of oils, wetting of solid surfaces, spreading, detergency, foaming, corrosion inhibition in fuel oils and equipment. | [8,120] |
| Mining | Heavy metal cleanup operations; Soil remediation; Flotation | Wetting and foaming, collectors and frothers, removal of metal ions from aqueous solutions, soil and sediments, heavy metals sequestrants, spreading, corrosion inhibition in oils. | [121] |
| Food | Emulsification and de-emulsification; Functional ingredient | Solubilisation of flavoured oils, control of consistency, emulsification, wetting agent, spreading, detergency, foaming, thickener. | [4] |
| Medicine | Microbiological; Pharmaceuticals and therapeutics | Anti-adhesive agents, antifungal agents, antibacterial agents, antiviral agents, vaccines, gene therapy, immunomodulatory molecules. | [20,122,123] |
| Agriculture | Biocontrol; Fertilisers | Wetting, dispersion, suspension of powdered pesticides and fertilisers, emulsification of pesticide solutions, facilitation of biocontrol mechanisms of microbes, plant pathogen elimination and increased bioavailability of nutrients for beneficial plant-associated microbes. | [124] |
| Cosmetics | Health and beauty products | Emulsification, foaming agents, solubilisation, wetting agents, cleansers, antimicrobial agents, mediators of enzyme action. | [5] |
| Cleaning | Washing detergents | Detergents and sanitisers for laundry, wetting, spreading, corrosion inhibition. | [3,5] |
| Textiles | Preparation of fibres; Dyeing and printing; Finishing of textiles | Wetting, penetration, solubilisation, emulsification, detergency and dispersion, wetting and emulsification in finishing formulations, softening. | [3,103] |
| Nanotechnology | Synthesis of nanoparticles | Emulsification, stabilisation. | [5,125] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030401
Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: Multifunctional Biomolecules of the 21st Century. International Journal of Molecular Sciences. 2016; 17(3):401. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030401
Chicago/Turabian StyleSantos, Danyelle Khadydja F., Raquel D. Rufino, Juliana M. Luna, Valdemir A. Santos, and Leonie A. Sarubbo. 2016. "Biosurfactants: Multifunctional Biomolecules of the 21st Century" International Journal of Molecular Sciences 17, no. 3: 401. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030401