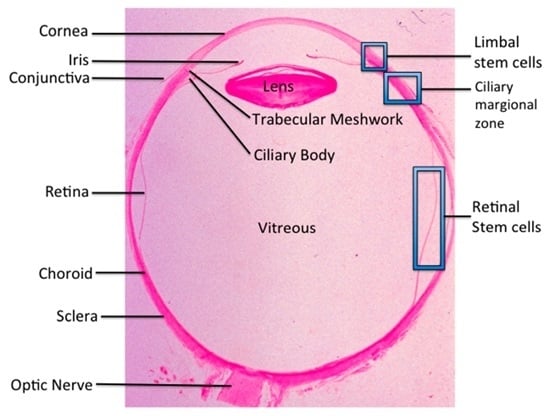
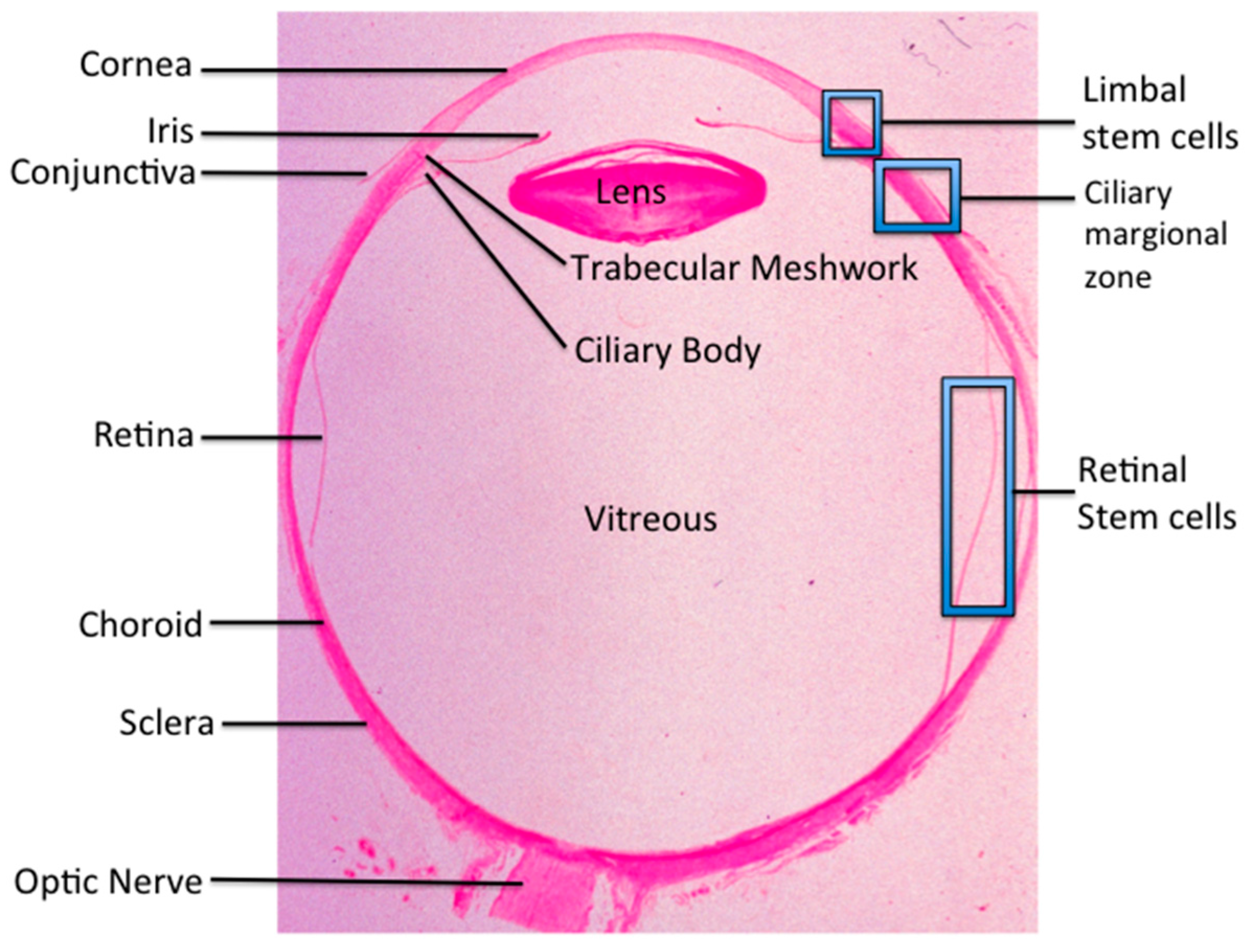
Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium
Abstract
:Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| RP | retinitis pigmentosa |
| RPE | retinal pigment epithelium |
| hESC | human embryonic stem cell |
| iPSC | induced pluripotent stem cell |
| LSC | limbal stem cell |
| LSCD | limbal stem cell deficiency |
| ABCB5 | ATP-binding cassette subfamily B member 5 |
| CEC | corneal epithelial cell |
| PAX6 | paired box protein 6 |
| AM | amniotic membrane |
| LAM | AM with live stromal cells |
| DAM | dead denuded AM |
| RGC | retinal ganglion cell |
| Ngn | neurogenin |
| RSC | retinal stem cell |
| CMZ | ciliary marginal zone |
| RPC | retinal progenitor cell |
| NSC | neural stem cell |
| MSC | mesenchymal stem cell |
| ADSC | adipose-derived stem cell |
| ESC | embryonic stem cell |
| CFH | complement factor H |
| FACS | fluorescence-activated cell sorting |
| qPCR | quantitative polymerase chain reaction |
| ACAID | anterior chamber altered immune deviation |
| IL | interleukin |
| MHC | major histocompatibility complex |
References
- Land, M.F.; Fernald, R.D. The evolution of eyes. Annu. Rev. Neurosci. 1992, 15, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Joseph, A.; Shanmuganathan, V.A.; Jones, R.E. Stem cell differentiation and the effects of deficiency. Eye 2003, 17, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–722. [Google Scholar] [PubMed]
- Pellegrini, G.; Ranno, R.; Stracuzzi, G.; Bondanza, S.; Guerra, L.; Zambruno, G.; Micali, G.; de Luca, M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 1999, 68, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; de Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kolli, S.; Li, D.Q.; de Paiva, C.S.; Pryzborski, S.; Dimmick, I.; Armstrong, L.; Figueiredo, F.C.; Lako, M. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells 2008, 26, 1609–1619. [Google Scholar] [PubMed]
- Schlotzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Eichner, R.; Bonitz, P.; Sun, T.T. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J. Cell Biol. 1984, 98, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M.; Gorivodsky, M.; Shtrom, S.; Grinberg, A.; Niehrs, C.; Morasso, M.I.; Westphal, H. DKK2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 2006, 133, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.T.; Hayashida, Y.; Blanco, G.; Kheirkah, A.; He, H.; Chen, S.Y.; Liu, C.Y.; Tseng, S.C. Down-regulation of PAX6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J. Pathol. 2008, 214, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xue, Y.; Lin, Y.; Zhang, X.; Xi, L.; Patel, S.; Cai, H.; Luo, J.; Zhang, M.; Zhang, M.; et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014, 511, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.; Ahmad, S.; Mudhar, H.S.; Meeny, A.; Lako, M.; Figueiredo, F.C. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 2014, 32, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Burillon, C.; Huot, L.; Justin, V.; Nataf, S.; Chapuis, F.; Decullier, E.; Damour, O. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Saghizadeh, M.; Ornelas, L.; Winkler, M.A.; Narwani, K.; Sahabian, A.; Funari, V.A.; Tang, J.; Spurka, L.; Punj, V.; et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl. Med. 2014, 3, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; Chen, Y.T.; He, H.; Tseng, D.Y.; Alonso, M.; Chen, S.Y.; Xi, X.; Tseng, S.C. Air exposure induced squamous metaplasia of human limbal epithelium. Investig. Ophthalmol. Vis. Sci. 2008, 49, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, T.; Dong, N.; Dong, F.; Fang, X.; Qu, Y.L.; Tan, Y.; Wu, H.; Liu, Z.; Li, W. Oxygen tension affects terminal differentiation of corneal limbal epithelial cells. J. Cell. Physiol. 2011, 226, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; He, H.; Kuo, C.L.; Tseng, S.C. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2007, 48, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Williams, A.; Waisbourd, M.; Iacovitti, L.; Katz, L.J. Stem cell therapy for glaucoma: Science or snake oil? Surv. Ophthalmol. 2015, 60, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Martin, K. Stem cell approaches to glaucoma: From aqueous outflow modulation to retinal neuroprotection. Prog. Brain Res. 2015, 220, 241–256. [Google Scholar] [PubMed]
- Sluch, V.M.; Zack, D.J. Stem cells, retinal ganglion cells and glaucoma. Dev. Ophthalmol. 2014, 53, 111–121. [Google Scholar] [PubMed]
- Du, Y.; Roh, D.S.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L.; Schuman, J.S. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O. The potential of stem cell research for the treatment of neuronal damage in glaucoma. Cell Tissue Res. 2013, 353, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann-Noor, A.H.; Vijay, S.; Limb, G.A.; Khaw, P.T. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov. Today 2010, 15, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Sluch, V.M.; Davis, C.H.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.Q.; et al. Differentiation of human escs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 2015, 5, 16595. [Google Scholar] [CrossRef] [PubMed]
- Kador, K.E.; Montero, R.B.; Venugopalan, P.; Hertz, J.; Zindell, A.N.; Valenzuela, D.A.; Uddin, M.S.; Lavik, E.B.; Muller, K.J.; Andreopoulos, F.M.; et al. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials 2013, 34, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef] [PubMed]
- Bermingham-McDonogh, O.; Reh, T.A. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron 2011, 71, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Hamon, A.; Roger, J.E.; Yang, X.J.; Perron, M. Muller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev. Dyn. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, W.; Zhuo, Y.; Yan, R.T.; Wang, S.Z. Using neurogenin to reprogram chick rpe to produce photoreceptor-like neurons. Investig. Ophthalmol. Vis. Sci. 2010, 51, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.T.; Li, X.; Wang, S.Z. Photoreceptor-like cells in transgenic mouse eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4766–4775. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.A. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis. Neurosci. 2000, 17, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Barthel, L.K.; Raymond, P.A. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 9310–9315. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Reh, T.A. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Cepko, C.L. Control of muller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 2000, 3, 873–880. [Google Scholar] [PubMed]
- Blackshaw, S.; Harpavat, S.; Trimarchi, J.; Cai, L.; Huang, H.; Kuo, W.P.; Weber, G.; Lee, K.; Fraioli, R.E.; Cho, S.H.; et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004, 2, E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T.A. Transgenic expression of the proneural transcription factor ascl1 in muller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13717–13722. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, S.; Glasgow, N.G.; Gibson, G.; Yang, X.; Shiber, C.E.; Funderburgh, J.; Watkins, S.; Johnson, J.W.; Schuman, J.S.; et al. Lgr5(+) amacrine cells possess regenerative potential in the retina of adult mice. Aging Cell 2015, 14, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Stenkamp, D.L. Neurogenesis in the fish retina. Int. Rev. Cytol. 2007, 259, 173–224. [Google Scholar] [PubMed]
- Lenkowski, J.R.; Raymond, P.A. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef] [PubMed]
- Fausett, B.V.; Goldman, D. A role for alpha1 tubulin-expressing muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial muller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed]
- Fimbel, S.M.; Montgomery, J.E.; Burket, C.T.; Hyde, D.R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 2007, 27, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Fausett, B.V.; Goldman, D. Ascl1a regulates muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microrna signalling pathway. Nat. Cell Biol. 2010, 12, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Fausett, B.V.; Gumerson, J.D.; Goldman, D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 2008, 28, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Zhao, X.F.; Goldman, D. Ascl1a/Dkk/β-catenin signaling pathway is necessary and glycogen synthase kinase-3β inhibition is sufficient for zebrafish retina regeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 15858–15863. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ramachandran, R.; Goldman, D. Hb-egf is necessary and sufficient for muller glia dedifferentiation and retina regeneration. Dev. Cell 2012, 22, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Zhao, X.F.; Goldman, D. Insm1a-mediated gene repression is essential for the formation and differentiation of muller glia-derived progenitors in the injured retina. Nat. Cell Biol. 2012, 14, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.P.; Roesch, K.; Cepko, C.L. Development and neurogenic potential of muller glial cells in the vertebrate retina. Prog. Retin. Eye Res. 2009, 28, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Roesch, K.; Jadhav, A.P.; Trimarchi, J.M.; Stadler, M.B.; Roska, B.; Sun, B.B.; Cepko, C.L. The transcriptome of retinal muller glial cells. J. Comp. Neurol. 2008, 509, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Ueki, Y.; Reardon, S.; Karl, M.O.; Georgi, S.; Hartman, B.H.; Lamba, D.A.; Reh, T.A. Genome-wide analysis of muller glial differentiation reveals a requirement for notch signaling in postmitotic cells to maintain the glial fate. PLoS ONE 2011, 6, e22817. [Google Scholar] [CrossRef] [PubMed]
- Das, A.V.; Mallya, K.B.; Zhao, X.; Ahmad, F.; Bhattacharya, S.; Thoreson, W.B.; Hegde, G.V.; Ahmad, I. Neural stem cell properties of muller glia in the mammalian retina: Regulation by Notch and wnt signaling. Dev. Biol. 2006, 299, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lewallen, M.; Chen, S.; Yu, W.; Zhang, N.; Xie, T. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013, 23, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, Y.; Deng, H. Direct lineage reprogramming: Strategies, mechanisms, and applications. Cell Stem Cell 2015, 16, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Pollak, J.; Wilken, M.S.; Ueki, Y.; Cox, K.E.; Sullivan, J.M.; Taylor, R.J.; Levine, E.M.; Reh, T.A. Ascl1 reprograms mouse muller glia into neurogenic retinal progenitors. Development 2013, 140, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Guan, Y.; Cui, L.; Song, J.; Gu, J.; Zhao, H.; Xu, L.; Lu, L.; Jin, Y.; Xu, G.T. Transplantation of rat embryonic stem cell-derived retinal progenitor cells preserves the retinal structure and function in rat retinal degeneration. Stem Cell Res. Ther. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nat. Neurosci. 2012, 15, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Light, A.C.; Zhu, Y.; Shi, J.; Saszik, S.; Lindstrom, S.; Davidson, L.; Li, X.; Chiodo, V.A.; Hauswirth, W.W.; Li, W.; et al. Organizational motifs for ground squirrel cone bipolar cells. J. Comp. Neurol. 2012, 520, 2864–2887. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.; Snellman, J.; Chen, S.; Li, W.; Zenisek, D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron 2013, 77, 516–527. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.B.; Long, S.S.; Fleming, H.; Li, W.; Fuerst, P.G. Dscam localization and function at the mouse cone synapse. J. Comp. Neurol. 2014, 522, 2609–2633. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, K.J.; Grunert, U.; Li, W. Processing of S-cone signals in the inner plexiform layer of the mammalian retina. Vis. Neurosci. 2014, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, J.; Zhao, C.; Wang, K.; Trood, E.; Buehler, J.; Weed, M.; Kasuga, D.; Bernstein, P.S.; Hughes, G.; et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch. Ophthalmol. 2011, 129, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, S. Stem cells and eye development. N. Engl. J. Med. 2011, 365, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010, 7, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, W.; Zhang, Y.; Wei, W.; Ambasudhan, R.; Xia, P.; Talantova, M.; Lin, T.; Kim, J.; Wang, X.; et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 8299–8304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Welsbie, D.S.; Yang, Z.; Ge, Y.; Mitchell, K.L.; Zhou, X.; Martin, S.E.; Berlinicke, C.A.; Hackler, L., Jr.; Fuller, J.; Fu, J.; et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. USA 2013, 110, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, K.J.; Maruotti, J.; Zack, D.J. Modeling retinal dystrophies using patient-derived induced pluripotent stem cells. Adv. Exp. Med. Biol. 2014, 801, 157–164. [Google Scholar] [PubMed]
- Fuller, J.A.; Shaw, G.C.; Bonnet-Wersinger, D.; Hansen, B.S.; Berlinicke, C.A.; Inglese, J.; Zack, D.J. A high content screening approach to identify molecules neuroprotective for photoreceptor cells. Adv. Exp. Med. Biol. 2014, 801, 773–781. [Google Scholar] [PubMed]
- Maruotti, J.; Sripathi, S.R.; Bharti, K.; Fuller, J.; Wahlin, K.J.; Ranganathan, V.; Sluch, V.M.; Berlinicke, C.A.; Davis, J.; Kim, C.; et al. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.; Grassmann, F.; Riolfi, J.; Weber, B.H. Tapping stem cells to target amd: Challenges and prospects. J. Clin. Med. 2015, 4, 282–303. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.S.; Bharti, K. Regenerating retinal pigment epithelial cells to cure blindness: A road towards personalized artificial tissue. Curr. Stem Cell Rep. 2015, 1, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V. Privilege revisited: An evaluation of the eye’s defence mechanisms. Eye 2009, 23, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. The induction of anterior chamber-associated immune deviation. Chem. Immunol. Allergy 2007, 92, 27–35. [Google Scholar] [PubMed]
- Enzmann, V.; Kaufmann, A.; Hollborn, M.; Wiedemann, P.; Gemsa, D.; Kohen, L. Effective chemokines and cytokines in the rejection of human retinal pigment epithelium (RPE) cell grafts. Transpl. Immunol. 1999, 7, 9–14. [Google Scholar] [CrossRef]
- Detrick, B.; Rodrigues, M.; Chan, C.C.; Tso, M.O.; Hooks, J.J. Expression of hla-dr antigen on retinal pigment epithelial cells in retinitis pigmentosa. Am. J. Ophthalmol. 1986, 101, 584–590. [Google Scholar] [CrossRef]
- Chan, C.C.; Detrick, B.; Nussenblatt, R.B.; Palestine, A.G.; Fujikawa, L.S.; Hooks, J.J. HLA-DR antigens on retinal pigment epithelial cells from patients with uveitis. Arch. Ophthalmol. 1986, 104, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Chan, C.C.; Sen, H.N.; Ferris, F.L.; Nussenblatt, R.B. Inflammatory mechanisms of age-related macular degeneration. Int. Ophthalmol. Clin. 2015, 55, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Percopo, C.M.; Hooks, J.J.; Shinohara, T.; Caspi, R.; Detrick, B. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J. Immunol. 1990, 145, 4101–4107. [Google Scholar] [PubMed]
- Benson, J.L.; Niederkorn, J.Y. The presence of donor-derived class ii-positive cells abolishes immune privilege in the anterior chamber of the eye. Transplantation 1991, 51, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Lucas, K.; Stein-Streilein, J. Retinal laser burn disrupts immune privilege in the eye. Am. J. Pathol. 2009, 174, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, B.; Tuo, J.; Shen, D.; Chen, P.; Li, Z.; Liu, X.; Ni, J.; Dagur, P.; Sen, H.N.; et al. Hypomethylation of the IL17RC promoter associates with age-related macular degeneration. Cell Rep. 2012, 2, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shen, D.; Patel, M.M.; Tuo, J.; Johnson, T.M.; Olsen, T.W.; Chan, C.C. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol. Int. 2011, 61, 528–535. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, H.; Goldberg, J.L.; Chen, S.; Li, W.; Xu, G.-T.; Li, W.; Zhang, K.; Nussenblatt, R.B.; Liu, Y.; Xie, T.; et al. Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium. Int. J. Mol. Sci. 2016, 17, 415. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030415
Ouyang H, Goldberg JL, Chen S, Li W, Xu G-T, Li W, Zhang K, Nussenblatt RB, Liu Y, Xie T, et al. Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium. International Journal of Molecular Sciences. 2016; 17(3):415. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030415
Chicago/Turabian StyleOuyang, Hong, Jeffrey L. Goldberg, Shuyi Chen, Wei Li, Guo-Tong Xu, Wei Li, Kang Zhang, Robert B. Nussenblatt, Yizhi Liu, Ting Xie, and et al. 2016. "Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium" International Journal of Molecular Sciences 17, no. 3: 415. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030415