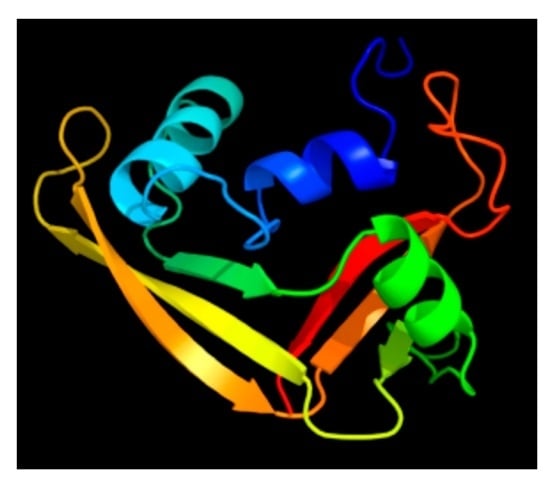
A Review of Ribonuclease 7’s Structure, Regulation, and Contributions to Host Defense
Abstract
:1. Introduction
2. Discovery and Characterization of the RNase 7 Peptide
3. RNase 7’s Bactericidal Mechanisms
4. RNase 7 Expression and Roles in Host Defense
5. RNase 7 Induction and Regulation
6. Regulation of RNase 7 by the Ribonuclease Inhibitor
7. Prospective
7.1. Does RNase 7 Play a Significant Role in Host Defense?
7.2. Does Altered RNase 7 Production Impact Infection Susceptibility?
7.3. What Is the Significance of RNase 7’s Catalytic Activity?
7.4. What Mechanisms Are essential for RNase 7’s Antimicrobial Properties?
7.5. What Molecular Processes Regulate RNase 7 Expression?
7.6. Will We Realize the Therapeutic Potential for RNase 7?
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol. 2003, 43, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Boix, E.; Nogues, M.V. Mammalian antimicrobial proteins and peptides: Overview on the RNase A superfamily members involved in innate host defence. Mol. Biosyst. 2007, 3, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Becknell, B.; Schwaderer, A.; Hains, D.S.; Spencer, J.D. Amplifying renal immunity: The role of antimicrobial peptides in pyelonephritis. Nat. Rev. Nephrol. 2015, 11, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dyer, K.D.; Rosenberg, H.F. Human RNase 7: A new cationic ribonuclease of the RNase A superfamily. Nucleic Acids Res. 2003, 31, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Beintema, J.J.; Kleineidam, R.G. The ribonuclease A superfamily: General discussion. Cell. Mol. Life Sci. 1998, 54, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Haigh, B.J.; Griffin, F.J.; Wheeler, T.T. The mammalian secreted RNases: Mechanisms of action in host defence. Innate Immun. 2013, 19, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S. The eight human “canonical” ribonucleases: Molecular diversity, catalytic properties, and special biological actions of the enzyme proteins. FEBS Lett. 2010, 584, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Beintema, J.J.; Zhang, J. The ribonuclease A superfamily of mammals and birds: Identifying new members and tracing evolutionary histories. Genomics 2005, 85, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Goo, S.M.; Cho, S. The expansion and functional diversification of the mammalian ribonuclease a superfamily epitomizes the efficiency of multigene families at generating biological novelty. Genome Biol. Evol. 2009, 5, 2124–2140. [Google Scholar] [CrossRef] [PubMed]
- Simanski, M.; Koten, B.; Schroder, J.M.; Glaser, R.; Harder, J. Antimicrobial RNases in cutaneous defense. J. Innate Immun. 2012, 4, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F. RNase A ribonucleases and host defense: An evolving story. J. Leukoc. Biol. 2008, 83, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schroder, J.M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002, 277, 46779–46784. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lin, Y.M.; Chang, T.W.; Wu, S.J.; Lee, Y.S.; Chang, M.D.; Chen, C.; Wu, S.H.; Liao, Y.D. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J. Biol. Chem. 2007, 282, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Koten, B.; Simanski, M.; Glaser, R.; Podschun, R.; Schroder, J.M.; Harder, J. RNase 7 contributes to the cutaneous defense against Enterococcus faecium. PLoS ONE 2009, 4, e6424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schwaderer, A.L.; Kline, J.; Spencer, J.D.; Kline, D.; Hains, D.S. Contribution of structural domains to the activity of ribonuclease 7 against uropathogenic bacteria. Antimicrob. Agents Chemother. 2013, 57, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Pulido, D.; Valle, J.; Nogues, M.V.; Andreu, D.; Boix, E. Ribonucleases as a host-defence family: Evidence of evolutionarily conserved antimicrobial activity at the N-terminus. Biochem. J. 2013, 456, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Becknell, B.; Eichler, T.E.; Beceiro, S.; Li, B.; Easterling, R.S.; Carpenter, A.R.; James, C.L.; McHugh, K.M.; Hains, D.S.; Partida-Sanchez, S.; Spencer, J.D. Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 2015, 87, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; Wang, H.; Bartz, J.; Kline, J.; Eichler, T.; DeSouza, K.R.; Sims-Lucas, S.; Baker, P.; Hains, D.S. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013, 83, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Boix, E.; Torrent, M.; Sanchez, D.; Nogues, M.V. The antipathogen activities of eosinophil cationic protein. Curr. Pharm. Biotechnol. 2008, 9, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Sanchez, D.; Buzon, V.; Nogues, M.V.; Cladera, J.; Boix, E. Comparison of the membrane interaction mechanism of two antimicrobial RNases: RNase 3/ECP and RNase 7. Biochim. Biophys. Acta 2009, 1788, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Badia, M.; Moussaoui, M.; Sanchez, D.; Nogues, M.V.; Boix, E. Comparison of human RNase 3 and RNase 7 bactericidal action at the Gram-negative and Gram-positive bacterial cell wall. FEBS J. 2010, 277, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Wu, S.J.; Chang, T.W.; Wang, C.F.; Suen, C.S.; Hwang, M.J.; Chang, M.D.; Chen, Y.T.; Liao, Y.D. Outer membrane protein I of Pseudomonas aeruginosa is a target of cationic antimicrobial peptide/protein. J. Biol. Chem. 2010, 285, 8985–8994. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Lin, Y.M.; Wang, C.F.; Liao, Y.D. Outer membrane lipoprotein Lpp is Gram-negative bacterial cell surface receptor for cationic antimicrobial peptides. J. Biol. Chem. 2012, 287, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; Dirosario, J.D.; McHugh, K.M.; McGillivary, G.; Justice, S.S.; Carpenter, A.R.; Baker, P.B.; Harder, J.; Hains, D.S. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011, 80, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Varoga, D.; Pufe, T.; Mentlein, R.; Kohrs, S.; Grohmann, S.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Expression and regulation of antimicrobial peptides in articular joints. Ann. Anat. 2005, 187, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Yeung, A.; Abedin, A.; Hopkinson, A.; Dua, H.S. Signalling pathways involved in ribonuclease-7 expression. Cell. Mol. Life Sci. 2011, 68, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Tam, C.; Chan, G.; Kim, J.H.; Evans, D.; Fleiszig, S. MicroRNA-762 is upregulated in human corneal epithelial cells in response to tear fluid and Pseudomonas aeruginosa antigens and negatively regulates the expression of host defense genes encoding RNase7 and ST2. PLoS ONE 2013, 8, e57850. [Google Scholar]
- Amatngalim, G.D.; van Wijck, Y.; de Mooij-Eijk, Y.; Verhoosel, R.M.; Harder, J.; Lekkerkerker, A.N.; Janssen, R.A.; Hiemstra, P.S. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein RNase 7. J. Immunol. 2015, 194, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Abtin, A.; Eckhart, L.; Mildner, M.; Ghannadan, M.; Harder, J.; Schroder, J.M.; Tschachler, E. Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase 7. J. Investig. Dermatol. 2009, 129, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Reithmayer, K.; Meyer, K.C.; Kleditzsch, P.; Tiede, S.; Uppalapati, S.K.; Glaser, R.; Harder, J.; Schroder, J.M.; Paus, R. Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Br. J. Dermatol. 2009, 161, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chassin, C.; Goujon, J.M.; Darche, S.; du Merle, L.; Bens, M.; Cluzeaud, F.; Werts, C.; Ogier-Denis, E.; Le Bouguenec, C.; Buzoni-Gatel, D.; et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 2006, 177, 4773–4784. [Google Scholar] [CrossRef] [PubMed]
- Simanski, M.; Dressel, S.; Glaser, R.; Harder, J. RNase 7 protects healthy skin from Staphylococcus aureus colonization. J. Investig. Dermatol. 2010, 130, 2836–2838. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; Eichler, T.; Wang, H.; Kline, J.; Justice, S.S.; Cohen, D.M.; Hains, D.S. An endogenous ribonuclease inhibitor regulates the antimicrobial activity of ribonuclease 7 in the human urinary tract. Kidney Int. 2014, 85, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Dressel, S.; Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Mrowietz, U.; Folster-Holst, R.; Proksch, E.; Schroder, J.M.; Schwarz, T.; et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J. Investig. Dermatol. 2010, 130, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, G.J.; Zeeuwen, P.L.; Kucharekova, M.; Pfundt, R.; van der Valk, P.G.; Blokx, W.; Dogan, A.; Hiemstra, P.S.; van de Kerkhof, P.C.; Schalkwijk, J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J. Investig. Dermatol. 2005, 125, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Skrygan, M.; Tomi, N.S.; Othlinghaus, N.; Brockmeyer, N.H.; Altmeyer, P.; Kreuter, A. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int. Arch. Allergy Immunol. 2008, 147, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Morig, A.; Neumann, B.; Proksch, E. Expression of antimicrobial peptides and toll-like receptors is increased in tinea and pityriasis versicolor. Mycoses 2014, 57, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Simanski, M.; Rademacher, F.; Schroder, L.; Schumacher, H.M.; Glaser, R.; Harder, J. IL-17A and IFN-γ synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PLoS ONE 2013, 8, e59531. [Google Scholar]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Gotz, F.; Peschel, A.; Schaller, M.; Schittek, B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Investig. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Burgey, C.; Kern, W.V.; Romer, W.; Sakinc, T.; Rieg, S. The innate defense antimicrobial peptides hBD3 and RNase7 are induced in human umbilical vein endothelial cells by classical inflammatory cytokines but not Th17 cytokines. Microbes Infect. 2015, 17, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Firat, Y.H.; Simanski, M.; Rademacher, F.; Schroder, L.; Brasch, J.; Harder, J. Infection of keratinocytes with Trichophytum rubrum induces epidermal growth factor-dependent RNase 7 and human beta-defensin-3 expression. PLoS ONE 2014, 9, e93941. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.; Menzel, N.; Dommisch, H.; Winter, J.; Jepsen, S.; Mutters, R. The stage of native biofilm formation determines the gene expression of human β-defensin-2, psoriasin, ribonuclease 7 and inflammatory mediators: A novel approach for stimulation of keratinocytes with in situ formed biofilms. Oral Microbiol. Immunol. 2008, 23, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Otri, A.M.; Mohammed, I.; Abedin, A.; Cao, Z.; Hopkinson, A.; Panjwani, N.; Dua, H.S. Antimicrobial peptides expression by ocular surface cells in response to Acanthamoeba castellanii: An in vitro study. Br. J. Ophthalmol. 2010, 94, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.A.; Haigis, M.C.; Raines, R.T. Ribonuclease inhibitor: Structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 349–374. [Google Scholar] [PubMed]
- Zasloff, M. The antibacterial shield of the human urinary tract. Kidney Int. 2013, 83, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrod, T.; Dalpke, A.H. Bacterial RNA: An underestimated stimulus for innate immune responses. J. Immunol. 2015, 195, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.A.; Rubin, J.; Moussaoui, M.; Pulido, D.; Nogues, M.V.; Venge, P.; Boix, E. Protein post-translational modification in host defense: The antimicrobial mechanism of action of human eosinophil cationic protein native forms. FEBS J. 2014, 281, 5432–5446. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becknell, B.; Spencer, J.D. A Review of Ribonuclease 7’s Structure, Regulation, and Contributions to Host Defense. Int. J. Mol. Sci. 2016, 17, 423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030423
Becknell B, Spencer JD. A Review of Ribonuclease 7’s Structure, Regulation, and Contributions to Host Defense. International Journal of Molecular Sciences. 2016; 17(3):423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030423
Chicago/Turabian StyleBecknell, Brian, and John David Spencer. 2016. "A Review of Ribonuclease 7’s Structure, Regulation, and Contributions to Host Defense" International Journal of Molecular Sciences 17, no. 3: 423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030423