Asymmetric Dimethylarginine versus Proton Pump Inhibitors Usage in Patients with Stable Coronary Artery Disease: A Cross-Sectional Study
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Mechanistic Considerations
3.2. Study Limitations
4. Materials and Methods
4.1. Patients
4.2. Procedure
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitors |
| ADMA | asymmetric dimethylarginine |
| ANCOVA | analysis of covariance |
| ANOVA | analysis of variance |
| CAD | coronary artery disease |
| CYP | cytochrome P450 |
| DDAH-1 | type 1 dimethylarginine dimethylaminohydrolase |
| ELISA | enzyme-linked immunosorbent assay |
| GFR | glomerular filtration rate |
| HDL | high-density lipoproteins |
| HO-1 | type 1 heme oxygenase |
| LDL | low-density lipoproteins |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NO | nitric oxide |
| PPI | proton pump inhibitors |
| PRMTs-I | type I protein-arginine N-methyltransferases |
| SD | standard deviation |
References
- Ghebremariam, Y.T.; LePendu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Blankenberg, S.; Lubos, E.; Lackner, K.J.; Rupprecht, H.J.; Espinola-Klein, C.; Jachmann, N.; Post, F.; Peetz, D.; Bickel, C.; et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: Results from the AtheroGene study. Circ. Res. 2005, 97, e53–e59. [Google Scholar] [CrossRef] [PubMed]
- Meinitzer, A.; Seelhorst, U.; Wellnitz, B.; Halwachs-Baumann, G.; Boehm, B.O.; Winkelmann, B.R.; März, W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study). Clin. Chem. 2007, 53, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.H.; Cho, L.; Brennan, D.M.; Hazen, S.L. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: Potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.M.; Maddox, T.M.; Wang, L.; Fihn, S.D.; Jesse, R.L.; Peterson, E.D.; Rumsfeld, J.S. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009, 301, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, D.N.; Gomes, T.; Ko, D.T.; Szmitko, P.E.; Austin, P.C.; Tu, J.V.; Henry, D.A.; Kopp, A.; Mamdani, M.M. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009, 180, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Charlot, M.; Ahlehoff, O.; Norgaard, M.L.; Jørgensen, C.H.; Sørensen, R.; Abildstrøm, S.Z.; Hansen, P.R.; Madsen, J.K.; Køber, L.; Torp-Pedersen, C.; et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: A nationwide cohort study. Ann. Intern. Med. 2010, 153, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Charlot, M.; Grove, E.L.; Hansen, P.R.; Olesen, J.B.; Ahlehoff, O.; Selmer, C.; Lindhardsen, J.; Madsen, J.K.; Køber, L.; Torp-Pedersen, C.; et al. Proton pump inhibitor use and risk of adverse cardiovascular events in aspirin treated patients with first time myocardial infarction: Nationwide propensity score matched study. BMJ 2011, 342, d2690. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.G.; Clare, R.; Pieper, K.S.; Nicolau, J.C.; Storey, R.F.; Cantor, W.J.; Mahaffey, K.W.; Angiolillo, D.J.; Husted, S.; Cannon, C.P.; et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: Insights from the platelet inhibition and patient outcomes trial. Circulation 2012, 125, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Jeevanantham, V.; Dawn, B.; Loke, Y.K. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Meta-analysis. Int. J. Cardiol. 2013, 167, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Chen, Y.T.; Ou, S.M.; Li, S.Y.; Chen, T.J.; Wang, S.J. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int. J. Cardiol. 2014, 177, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Cattaneo, M.; Collet, J.P.; Andreotti, F.; Lip, G.Y.; Verheugt, F.W.; Huber, K.; Grove, E.L.; Morais, J.; Husted, S.; et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur. Heart J. 2013, 34, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Washam, J.B.; Jones, W.S.; Halim, S.A.; Hasselblad, V.; Mayer, S.B.; Heidenfelder, B.L.; Dolor, R.J. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: Systematic review. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ghebremariam, Y.T.; Cooke, J.P.; Khan, F.; Thakker, R.N.; Chang, P.; Shah, N.H.; Nead, K.T.; Leeper, N.J. Proton pump inhibitors and vascular function: A prospective cross-over pilot study. Vasc. Med. 2015, 20, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gill, P.S.; Chabrashvili, T.; Onozato, M.L.; Raggio, J.; Mendonca, M.; Dennehy, K.; Li, M.; Modlinger, P.; Leiper, J.; et al. Isoform-specific regulation by NG,NG-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ. Res. 2007, 101, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Ingelsson, E.; Kumar, J.; Syvänen, A.C.; Axelsson, T.; Teerlink, T. Genetic variation in the dimethylarginine dimethylaminohydrolase 1 gene (DDAH1) is related to asymmetric dimethylarginine (ADMA) levels, but not to endothelium-dependent vasodilation. Vasc. Med. 2013, 18, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, I.; Kleber, M.E.; Lyytikäinen, L.P.; Hernesniemi, J.A.; Mäkelä, K.M.; Oksala, N.; Laaksonen, R.; Pilz, S.; Tomaschitz, A.; Silbernagel, G.; et al. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. Eur. Heart J. 2014, 35, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lüneburg, N.; Lieb, W.; Zeller, T.; Chen, M.H.; Maas, R.; Carter, A.M.; Xanthakis, V.; Glazer, N.L.; Schwedhelm, E.; Seshadri, S.; et al. Genome-wide association study of l-arginine and dimethylarginines reveals novel metabolic pathway for symmetric dimethylarginine. Circ. Cardiovasc. Genet. 2014, 7, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Delles, C.; Schneider, M.P.; John, S.; Gekle, M.; Schmieder, R.E. Angiotensin converting enzyme inhibition and angiotensin ii AT1-receptor blockade reduce the levels of asymmetrical NG,NG-dimethylarginine in human essential hypertension. Am. J. Hypertens. 2002, 15, 590–593. [Google Scholar] [CrossRef]
- Hetzel, S.; DeMets, D.; Schneider, R.; Borzak, S.; Schneider, W.; Serebruany, V.; Schröder, H.; Hennekens, C.H. Aspirin increases nitric oxide formation in chronic stable coronary disease. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Mikhailidis, D.P.; Rizzo, M.; Lip, G.Y.; Kees Hovingh, G.; Kastelein, J.J.; Kalinowski, L.; Rysz, J.; et al. A systematic review and meta-analysis of the effect of statins on plasma asym metric dimethylarginine concentrations. Sci. Rep. 2015, 5, 9902. [Google Scholar] [CrossRef] [PubMed]
- Krempl, T.K.; Maas, R.; Sydow, K.; Meinertz, T.; Böger, R.H.; Kähler, J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur. Heart J. 2005, 26, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Lenzen, H.; Hanefeld, C.; Bartling, A.; Osterziel, K.J.; Goudeva, L.; Schmidt-Lucke, C.; Kusus, M.; Maas, R.; Schwedhelm, E.; et al. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: Results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am. Heart J. 2006, 152. [Google Scholar] [CrossRef] [PubMed]
- Napora, M.; Graczykowska, A.; Próchniewska, K.; Zdrojewski, Z.; Całka, A.; Górny, J.; Stompór, T. Relationship between serum asymmetric dimethylarginine and left ventricular structure and function in patients with end‑stage renal disease treated with hemodialysis. Pol. Arch. Med. Wewn. 2012, 122, 226–234. [Google Scholar] [PubMed]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Grosser, N.; Waltke, C.; Schulz, S.; Erdmann, K.; Domschke, W.; Schröder, H.; Pohle, T. Beyond gastric acid reduction: Proton pump inhibitors induce heme oxygenase-1 in gastric and endothelial cells. Biochem. Biophys. Res. Commun. 2006, 345, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Sydow, K.; Borlak, J.; Thum, T.; Lenzen, H.; Schubert, B.; Tsikas, D.; Bode-Böger, S.M. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: Involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000, 87, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.; Kiefte-de Jong, J.C.; Eijgelsheim, M.; Franco, O.H.; Kuipers, E.J.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am. J. Kidney Dis. 2015, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.E.; Ell, C.; Galmiche, J.P.; Fiocca, R.; Hatlebakk, J.G.; Hasselgren, B.; Långström, G.; Jahreskog, M.; Eklund, S.; Lind, T.; et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: Data from the SOPRAN and LOTUS studies. Aliment. Pharmacol. Ther. 2015, 41, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Würtz, M.; Grove, E.L.; Kristensen, S.D.; Hvas, A.M. The antiplatelet effect of aspirin is reduced by proton pump inhibitors in patients with coronary artery disease. Heart 2010, 96, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, A.B.; Sakizlis, G.N.; Nasothimiou, E.G.; Anastasopoulou, I.; Anastasakou, E.; Kotsi, P.; Karafoulidou, A.; Stergiou, G.S. Do proton pump inhibitors attenuate the effect of aspirin on platelet aggregation? A randomized crossover study. J. Cardiovasc. Pharmacol. 2009, 54, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Uno, T.; Niioka, T.; Hayakari, M.; Yasui-Furukori, N.; Sugawara, K.; Tateishi, T. Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur. J. Clin. Pharmacol. 2007, 63, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Barter, Z.E.; Tucker, G.T.; Rowland-Yeo, K. Differences in cytochrome p450-mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modelling. Clin. Pharmacokinet. 2013, 52, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Krajčíová, L.; Petrovič, R.; Déžiová, L.; Chandoga, J.; Turčáni, P. Frequency of selected single nucleotide polymorphisms influencing the warfarin pharmacogenetics in Slovak population. Eur. J. Haematol. 2014, 93, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kruszelnicka, O.; Surdacki, A.; Golay, A. Differential associations of angiographic extent and severity of coronary artery disease with asymmetric dimethylarginine but not insulin resistance in non-diabetic men with stable angina: A cross-sectional study. Cardiovasc. Diabetol. 2013, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Kruszelnicka-Kwiatkowska, O.; Surdacki, A.; Goldsztajn, P.; Matysek, J.; Piwowarska, W.; Golay, A. Relationship between hyperinsulinemia and angiographically defined coronary atherosclerosis in non-diabetic men. Diabetes Metab. 2002, 28, 305–309. [Google Scholar] [PubMed]
- Sullivan, D.R.; Marwick, T.H.; Freedman, S.B. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am. Heart J. 1990, 119, 1262–1267. [Google Scholar] [CrossRef]
| Characteristic | Patients on PPI (n = 53) | Patients without PPI (n = 75) | p-Value |
|---|---|---|---|
| Age (years) | 59 ± 11 | 56 ± 10 | 0.12 |
| Body-mass index (kg/m2) | 27.7 ± 3.6 | 27.4 ± 3.5 | 0.6 |
| History of current smoking, n (%) | 16 (30%) | 20 (27%) | 0.8 |
| Multivessel CAD, n (%) | 41 (77%) | 54 (72%) | 0.6 |
| CAD extent score | 31 (21–44) | 28 (19–40) | 0.5 |
| Left ventricular ejection fraction (%) | 70 ± 7 | 68 ± 6 | 0.2 |
| Hypertension, n (%) | 43 (80%) | 56 (75%) | 0.4 |
| Mean blood pressure (mm Hg) | 96 ± 11 | 95 ± 10 | 0.7 |
| Estimated GFR (mL/min per 1.73 m2) | 69 ± 9 | 72 ± 11 | 0.09 |
| LDL cholesterol (mmol/L) | 2.8 ± 0.7 | 2.8 ± 0.6 | 0.8 |
| HDL cholesterol (mmol/L) | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.2 |
| Triglycerides (mmol/L) | 1.4 ± 0.6 | 1.5 ± 0.7 | 0.3 |
| Glucose (mmol/L) | 5.8 ± 0.9 | 5.7 ± 0.8 | 0.5 |
| High-sensitivity C-reactive protein (mg/L) | 1.9 (1.1–4.0) | 1.8 (1.0–3.8) | 0.8 |
| ADMA before Admission (µmol/L) | p-Value | ||
|---|---|---|---|
| PPI Users (n = 53) | PPI Non-Users (n = 75) | ||
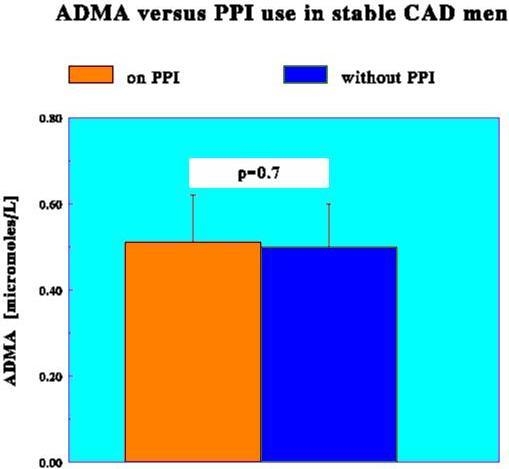
| All CAD subjects, n = 128 | 0.51 ± 0.11 | 0.50 ± 0.10 | 0.7 |
| History of current smoking | |||
| Yes, n = 36 | 0.51 ± 0.11 | 0.50 ± 0.10 | 0.4 |
| No, n = 92 | 0.51 ± 0.10 | 0.51 ± 0.11 | 0.8 |
| Severity of angiographic CAD | |||
| One-vessel disease, n = 33 | 0.48 ± 0.10 | 0.49 ± 0.10 | 0.7 |
| Multivessel disease, n = 95 | 0.52 ± 0.11 | 0.51 ± 0.11 | 0.9 |
| Extent of angiographic CAD | |||
| Sullivan extent score ≤ 29, n = 65 | 0.48 ± 0.09 | 0.49 ± 0.10 | 0.6 |
| Sullivan extent score > 29, n = 63 | 0.54 ± 0.11 | 0.52 ± 0.10 | 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszelnicka, O.; Świerszcz, J.; Bednarek, J.; Chyrchel, B.; Surdacki, A.; Nessler, J. Asymmetric Dimethylarginine versus Proton Pump Inhibitors Usage in Patients with Stable Coronary Artery Disease: A Cross-Sectional Study. Int. J. Mol. Sci. 2016, 17, 454. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040454
Kruszelnicka O, Świerszcz J, Bednarek J, Chyrchel B, Surdacki A, Nessler J. Asymmetric Dimethylarginine versus Proton Pump Inhibitors Usage in Patients with Stable Coronary Artery Disease: A Cross-Sectional Study. International Journal of Molecular Sciences. 2016; 17(4):454. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040454
Chicago/Turabian StyleKruszelnicka, Olga, Jolanta Świerszcz, Jacek Bednarek, Bernadeta Chyrchel, Andrzej Surdacki, and Jadwiga Nessler. 2016. "Asymmetric Dimethylarginine versus Proton Pump Inhibitors Usage in Patients with Stable Coronary Artery Disease: A Cross-Sectional Study" International Journal of Molecular Sciences 17, no. 4: 454. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040454