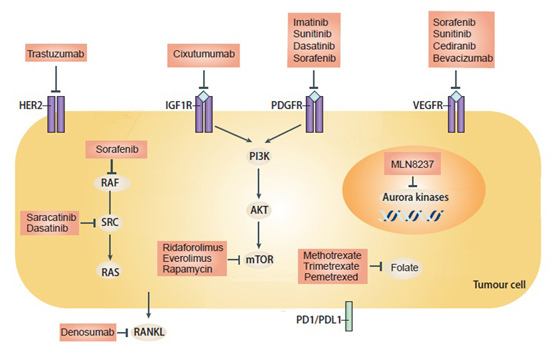
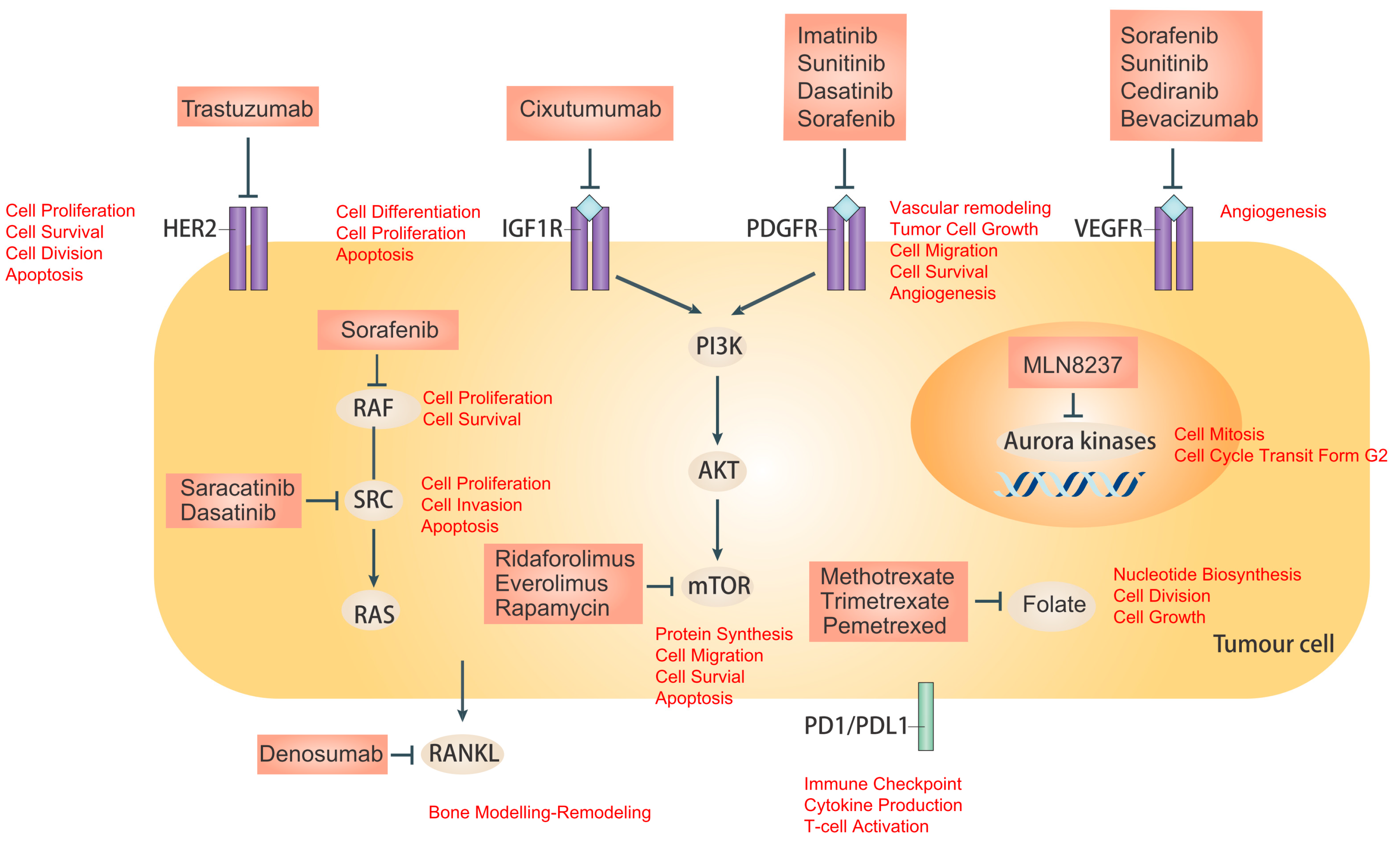
Present Advances and Future Perspectives of Molecular Targeted Therapy for Osteosarcoma
Abstract
:1. Introduction
2. Immunomodulators
2.1. Interferons
2.2. GM-CSF
2.3. Mifamurtide
3. Tyrosine Kinase Receptor Inhibitors
3.1. Receptor Tyrosine Kinases (RTKs)
3.2. Insulin-Like Growth Factor Receptor (IGF-R)
3.3. Platelet-Derived Growth Factor/Receptors (PDGF/PDGFR)
3.4. Vascular Endothelial Growth Factor (VEGF)
3.5. Human Epidermal Growth Factor Receptor 2 (HER-2)
4. Intracellular Signaling Inhibitors
4.1. Steroid Receptor Co-Activator (Src)
4.2. The Mammalian Target of Rapamycin (mTOR)
4.3. Aurora Kinase
5. Future Perspectives
5.1. Rank Inhibitors
5.2. Programmed Cell Death 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1) Pathway
5.3. MicroRNAs
5.4. Anti-Folate
5.5. Improved Delivery Systems
5.5.1. Doxorubicin Conjugate
5.5.2. Nanoparticle Albumin-Bound (Nab) Paclitaxel
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OS | Osteosarcoma |
| SRC | Steroid receptor co-activator |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| RB1 | Retinoblastoma 1 |
| TP53 | Tumor protein p53 |
| APEX1 | Apurinic/Apyrimidinic exonuclease 1 |
| BMPR2 | Bone morphogenetic protein type II receptor |
| HMGB1 | High mobility group box1 |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| OPG | Osteoprotegerin |
| RTKs | Receptor tyrosine kinases |
| PDGFR | Platelet-derived growth factor receptor |
| VEGFR | Vascular endothelial growth factor receptor |
| IFNs | Interferons |
| MAP | Methotrexate, doxorubicin, and cisplatin |
| EFS | Event-free survival |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| MDP | Muramyl dipeptide |
| MTP-PE | Muramyl tripeptide phosphatidyl ethanolamine |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| PI3 | Phosphatidylinositol 3 |
| Erk | Extracellular signal regulated kinase |
| IGF-R | Insulin-Like Growth Factor Receptor |
| IGF | Insulin-Like Growth Factor |
| MAPK | Mitogen-activated protein kinase |
| PDGF/PDGFR | Platelet-Derived Growth Factor/receptors |
| RCC | Renal cell carcinoma |
| GIST | Gastrointestinal stromal tumor |
| FLT-3 | Fms-related tyrosine kinase-3 |
| HCC | Hepatocellular carcinoma |
| CML | Chronic myeloid leukemia |
| ALL | Acute lymphoblastic leukemia |
| AURK | Aurora Kinase |
| ROS | Reactive Oxygen Species |
| AMPK | 5′ Adenosine monophosphate-activated protein kinase |
| RANK/RANKL | Receptor activator of nuclear factor-κB/Ligand |
| ROCK1 | Rho-associated, coiled-coil-containing protein kinase 1 |
| NK | Natural killer |
| nab | Nanoparticle albumin-bound |
| MBC | Metastatic breast cancer |
| TS | Thymidylate synthase |
| GARFT | Glycinamide ribonucleotide formyl transferase |
References
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National cancer data base report. Clin. Orthop. Relat. Res. 2007, 459, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.B. Osteosarcomagenesis: Modeling cancer initiation in the mouse. Sarcoma 2011, 2011, 694136. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Saha, K.; Banerjee, A.; Jash, D. Osteosarcoma relapse as pleural metastasis. South Asian J. Cancer 2013, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Chen, W.M.; Chen, C.F.; Lee, O.K.; Haung, C.K.; Chen, H.; Chen, T.H. Primary osteogenic sarcoma with pulmonary metastasis: Clinical results and prognostic factors in 91 patients. Jpn. J. Clin. Oncol. 2009, 39, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, M.; Schäfer, B.W. Targets for cancer therapy in childhood sarcomas. Cancer Treat. Rev. 2010, 36, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Ding, L.; Mardis, E.E.R.; Wilson, R.K.R.; Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell. Rep. 2014, 7, 104–112. [Google Scholar]
- Sampson, V.B.; Gorlick, R.; Kamara, D.; Anders Kolb, E. A review of targeted therapies evaluated by the pediatric preclinical testing program for osteosarcoma. Front. Oncol. 2013, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M. Prognostic Factors for Osteosarcoma Patients. In Osteosarcoma; Springer Japan: Tokyo, Japan, 2016; pp. 73–79. [Google Scholar]
- Morrow, J.; Khanna, C. Osteosarcoma genetics and epigenetics: Emerging biology and candidate therapies. Crit. Rev. Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Kansara, M.; Leong, H.S.; Lin, D.M.; Popkiss, S.; Pang, P.; Garsed, D.W.; Walkley, C.R.; Cullinane, C.; Ellul, J.; Haynes, N.M.; et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J. Clin. Investig. 2013, 123, 5351–5360. [Google Scholar] [CrossRef] [PubMed]
- Chandar, N.; Billig, B.; McMaster, J.; Novak, J. Inactivation of p53 gene in human and murine osteosarcoma cells. Br. J. Cancer 1992, 65, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, D.; Cogdell, D.; Du, X. APEX1 gene amplification and its protein overexpression in osteosarcoma: Correlation with recurrence, metastasis, and survival. Technol. Cancer Res. Treat. 2010, 9, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ishikawa, T.; Sugihara, E.; Kuninaka, S.; Miyamoto, T.; Mabuchi, Y.; Matsuzaki, Y.; Tsunoda, T.; Miya, F.; Morioka, H.; et al. c-MYC overexpression with loss of Ink4a/Arf transforms bone marrow stromal cells into osteosarcoma accompanied by loss of adipogenesis. Oncogene 2010, 29, 5687–5699. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Stoico, G.; Michelacci, F.; Pasello, M.; Scionti, I.; Remondini, D.; Castellani, G.C.; Fanelli, M.; Scotlandi, K.; Picci, P.; et al. Mechanisms of gene amplification and evidence of coamplification in drug-resistant human osteosarcoma cell lines. Genes Chromosomes Cancer 2009, 48, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.G.; Kandil, M.; Asaad, N.Y.; Dawoud, M.M.; Shahin, A.A.; Abd Eldayem, A.F. The prognostic role of Ezrin and HER2/neu expression in osteosarcoma. Appl. Immunohistochem. Mol. Morphol. 2015, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhao, J.; Liu, H.; Zhou, G.; Zhang, W.; Xu, X.; Zheng, M. HMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcoma. Tumor Biol. 2014, 35, 12265–12274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, P.; La Marca, F.; Than, K.; Rahman, S.; Lin, C.Y. Bone formation induced by BMP-2 in human osteosarcoma cells. Int. J. Oncol. 2013, 43, 1095–1102. [Google Scholar] [PubMed]
- Tarhini, A.A.; Kirkwood, J.M. How much of a good thing? What duration for interferon alfa-2b adjuvant therapy? J. Clin. Oncol. 2012, 30, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; Loggers, E.T.; Rodler, E.T.; Yee, C.; Jones, R.L. Immune-based therapies for sarcoma. Sarcoma 2011, 2011, 438940. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.R.; Smeland, S.; Bauer, H.C.F.; Saeter, G.; Strander, H. Interferon-alpha as the only adjuvant treatment in high-grade osteosarcoma: Long term results of the Karolinska Hospital series. Acta Oncol. 2005, 44, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Smeland, S.; Whelan, J.; Marina, N.; Hook, J.; Jovic, G.; Krailo, M.D.; Butterfass-Bahloul, T.; Kuhne, T.; Eriksson, M.; et al. MAP plus maintenance pegylated interferon α-2b (MAPIfn) versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 “good response” randomization. ASCO Meet. Abstr. 2013, 31, LBA10504. [Google Scholar]
- Kaufman, H.; Ruby, C.; Hughes, T.; Slingluff, C. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kato, M.; Kikuchi, A.; Hanada, R.; Koh, K. Delayed short-term administration of granulocyte colony-stimulating factor is a good mobilization strategy for harvesting autologous peripheral blood stem cells in pediatric patients with solid tumors. Pediatr. Transplant. 2013, 17, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Markovic, S.N.; Sloan, J.A.; Clawson, M.L.; Wylam, M.; Arndt, C.A.S.; Smithson, W.A.; Burch, P.; Gornet, M.; Rahman, E. Aerosol granulocyte macrophage-colony stimulating factor: A low toxicity, lung-specific biological therapy in patients with lung metastases. Clin. Cancer Res. 1999, 5, 2316–2323. [Google Scholar] [PubMed]
- Arndt, C.A.S.; Koshkina, N.V.; Inwards, C.Y.; Hawkins, D.S.; Krailo, M.D.; Villaluna, D.; Anderson, P.M.; Goorin, A.M.; Blakely, M.L.; Bernstein, M.; et al. Inhaled granulocyte-macrophage colony stimulating factor for first pulmonary recurrence of osteosarcoma: Effects on disease-free survival and immunomodulation. a report from the Children’s Oncology Group. Clin. Cancer Res. 2010, 16, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Heymann, M.-F.; Stresing, V.; Mori, K.; Rédini, F.; Heymann, D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers 2013, 5, 591–616. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Mifamurtide: A review of its use in the treatment of osteosarcoma. Pediatr. Drugs 2010, 12, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Tomaras, M.; McConnell, K. Mifamurtide in osteosarcoma—A practical review. Drugs Today 2010, 46, 327–337. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G.; Kurzman, I.D.; Rosenthal, R.C.; Smith, B.W.; Manley, P.A.; Roush, J.K.; Howard, P.E. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J. Natl. Cancer Inst. 1989, 81, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Kleinerman, E.S.; Krailo, M.D.; Chen, Z.; Betcher, D.L.; Healey, J.H.; Conrad, E.U.; Nieder, M.L.; Weiner, M.A.; Wells, R.J.; et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma. Cancer 2009, 115, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Meyers, P.; Kleinerman, E.; Venkatakrishnan, K.; Hughes, D.P.; Herzog, C.; Huh, W.; Sutphin, R.; Vyas, Y.M.; Shen, V.; et al. Mifamurtide in metastatic and recurrent osteosarcoma: A patient access study with pharmacokinetic, pharmacodynamic, and safety assessments. Pediatr. Blood Cancer 2014, 61, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.; Egas-Bejar, D.; Anderson, P. New treatment options for nonmetastatic osteosarcoma: Focus on mifamurtide in adolescents. Clin. Oncol. Adolesc. Young Adults 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Versleijen-Jonkers, Y.M.H.; Boerman, O.C.; van der Graaf, W.T.A. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: Current hurdles and future perspectives. Biochim. Biophys. Acta 2014, 1845, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell. 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Malempati, S.; Weigel, B.; Ingle, A.M.; Ahern, C.H.; Carroll, J.M.; Roberts, C.T.; Reid, J.M.; Schmechel, S.; Voss, S.D.; Cho, S.Y.; et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Weigel, B.; Malempati, S.; Reid, J.M.; Voss, S.D.; Cho, S.Y.; Chen, H.X.; Krailo, M.; Villaluna, D.; Adamson, P.C.; Blaney, S.M. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2014, 61, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Tap, W.D.; Qin, L.-X.; Livingston, M.B.; Undevia, S.D.; Chmielowski, B.; Agulnik, M.; Schuetze, S.; Reed, D.R.; Okuno, S.H.; et al. A phase II multicenter study of the IGF-1 receptor antibody cixutumumab (A12) and the mTOR inhibitor temsirolimus (TEM) in patients (pts) with refractory IGF-1R positive (+) and negative (–) bone and soft tissue sarcomas (STS). ASCO Meet. Abstr. 2012, 30, 10003. [Google Scholar]
- Schwartz, G.K.; Tap, W.D.; Qin, L.-X.; Livingston, M.B.; Undevia, S.D.; Chmielowski, B.; Agulnik, M.; Schuetze, S.M.; Reed, D.R.; Okuno, S.H.; et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2013, 14, 371–382. [Google Scholar] [CrossRef]
- Wagner, L.M.; Fouladi, M.; Ahmed, A.; Krailo, M.D.; Weigel, B.; DuBois, S.G.; Doyle, L.A.; Chen, H.; Blaney, S.M. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Bernstein, M.L.; Pappo, A.; Schultz, K.R.; Krailo, M.; Blaney, S.M.; Adamson, P.C. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: A Children’s Oncology Group study. Pediatr. Blood Cancer 2008, 50, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.G.; Shusterman, S.; Ingle, A.M.; Ahern, C.H.; Reid, J.M.; Wu, B.; Baruchel, S.; Glade-Bender, J.; Ivy, P.; Grier, H.E.; et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: A children’s oncology group study. Clin. Cancer Res. 2011, 17, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Glade Bender, J.L.; Adamson, P.C.; Reid, J.M.; Xu, L.; Baruchel, S.; Shaked, Y.; Kerbel, R.S.; Cooney-Qualter, E.M.; Stempak, D.; Chen, H.X.; et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children’s Oncology Group Study. J. Clin. Oncol. 2008, 26, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015, 16, 98–107. [Google Scholar] [CrossRef]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Available online: http://clinicaltrials.gov (accessed on 2 February 2016).
- Hassan, S.E.; Bekarev, M.; Kim, M.Y.; Lin, J.; Piperdi, S.; Gorlick, R.; Geller, D.S. Cell surface receptor expression patterns in osteosarcoma. Cancer 2012, 118, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.E.; Sorensen, P.H.B. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin. Ther. Targets 2012, 16, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Kuijjer, M.L.; Peterse, E.F.P.; van den Akker, B.E.W.M.; Briaire-de Bruijn, I.H.; Serra, M.; Meza-Zepeda, L.A.; Myklebost, O.; Hassan, A.B.; Hogendoorn, P.C.W.; et al. IR/IGF1R signaling as potential target for treatment of high-grade osteosarcoma. BMC Cancer 2013, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Han, X.-D.; Qiu, Y.; Xiong, J.; Yu, Y.; Wang, B.; Zhu, Z.-Z.; Qian, B.-P.; Chen, Y.-X.; Wang, S.-F.; et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J. Surg. Oncol. 2012, 105, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Burrow, S.; Andrulis, I.L.; Pollak, M.; Bell, R.S. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J. Surg. Oncol. 1998, 69, 21–27. [Google Scholar] [CrossRef]
- Wang, Y.L.Y.; Lipari, P.; Wang, X.Y.; Hailey, J.; Liang, L.Z.; Ramos, R.; Liu, M.; Pachter, J.A.; Bishop, W.R.; Wang, Y. A fully human insulin-like growth factor-I receptor antibody SCH 717454 (Robatumumab) has antitumor activity as a single agent and in combination with cytotoxics in pediatric tumor xenografts. Mol. Cancer Ther. 2010, 9, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol. Cancer Ther. 2014, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chesebrough, J.W.; Cartlidge, S.A.; Ricketts, S.A.; Incognito, L.; Veldman-Jones, M.; Blakey, D.C.; Tabrizi, M.; Jallal, B.; Trail, P.A.; et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011, 71, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Kolb, E.A.; Gorlick, R.; Lock, R.; Carol, H.; Morton, C.L.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Billups, C.; et al. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr. Blood Cancer 2011, 56, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Leeman, E.; Carnes, D.C.; Graves, D.T. Human osteoblasts synthesize and respond to platelet-derived growth factor. Am. J. Physiol. 1991, 261, C348–C354. [Google Scholar] [PubMed]
- Sulzbacher, I.; Birner, P.; Trieb, K.; Träxler, M.; Lang, S.; Chott, A. Expression of platelet-derived growth factor-AA is associated with tumor progression in osteosarcoma. Mod. Pathol. 2003, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, F.; Tamborini, E.; Negri, T.; Pastore, E.; Ferrari, A.; Luksch, R.; Casanova, M.; Pierotti, M.A.; Bellani, F.F.; Pilotti, S. Evidence for activation of KIT, PDGFRα, and PDGFRβ receptors in the Ewing sarcoma family of tumors. Cancer 2007, 109, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Sulzbacher, I.; Birner, P.; Dominkus, M.; Pichlhofer, B.; Mazal, P.R. Expression of platelet-derived growth factor-alpha receptor in human osteosarcoma is not a predictor of outcome. Pathology 2010, 42, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Takemoto, A.; Takami, M.; Oh-Hara, T.; Fujita, N. Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci. 2014, 105, 983–988. [Google Scholar] [CrossRef] [PubMed]
- McGary, E.C.; Weber, K.; Mills, L.; Doucet, M.; Lewis, V.; Lev, D.C.; Fidler, I.J.; Bar-Eli, M. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI. Clin. Cancer Res. 2002, 8, 3584–3591. [Google Scholar]
- Kubo, T.; Piperdi, S.; Rosenblum, J.; Antonescu, C.R.; Chen, W.; Kim, H.S.; Huvos, A.G.; Sowers, R.; Meyers, P.A.; Healey, J.H.; et al. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer 2008, 112, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Gobin, B.; Moriceau, G.; Ory, B.; Charrier, C.; Brion, R.; Blanchard, F.; Redini, F.; Heymann, D. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS ONE 2014, 9, e90795. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M.; Courtright, J.; Houghton, P.J.; Morton, C.L.; Kolb, E.A.; Lock, R.; Tajbakhsh, M.; Reynolds, C.P.; Keir, S.T.; Wu, J.; et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr. Blood Cancer 2008, 51, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.R.; Arlt, M.J.; Kuzmanov, A.; Born, W.; Fuchs, B. Sunitinib malate (SU-11248) reduces tumour burden and lung metastasis in an intratibial human xenograft osteosarcoma mouse model. Am. J. Cancer Res. 2015, 5, 2156–2168. [Google Scholar] [PubMed]
- Rivera-Valentin, R.K.; Zhu, L.; Hughes, D.P.M. Bone sarcomas in pediatrics: Progress in our understanding of tumor biology and implications for therapy. Pediatr. Drugs 2015, 17, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, P.; Zhang, W.; Gorlick, R.; Kolb, E. Activity of dasatinib in human osteosarcoma. Cancer Res. 2007, 67, 3255. [Google Scholar]
- Guan, H.; Zhou, Z.; Wang, H.; Jia, S.-F.; Liu, W.; Kleinerman, E.S. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing’s sarcoma growth in a xenograft mouse model. Clin. Cancer Res. 2005, 11, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Ługowska, I.; Woźniak, W.; Klepacka, T.; Michalak, E.; Szamotulska, K. A prognostic evaluation of vascular endothelial growth factor in children and young adults with osteosarcoma. Pediatr. Blood Cancer 2011, 57, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-W.; Wu, T.-Y.; Yi, X.; Ren, W.-P.; Zhou, Z.-B.; Sun, Y.-Q.; Zhang, C.-Q. Prognostic significance of VEGF expression in osteosarcoma: A meta-analysis. Tumour Biol. 2014, 35, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Niswander, L.M.; Kim, S.Y. Stratifying osteosarcoma: Minimizing and maximizing therapy. Curr. Oncol. Rep. 2010, 12, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Scharf, V.F.; Farese, J.P.; Coomer, A.R.; Milner, R.J.; Taylor, D.P.; Salute, M.E.; Chang, M.N.; Neal, D.; Siemann, D.W. Effect of bevacizumab on angiogenesis and growth of canine osteosarcoma cells xenografted in athymic mice. Am. J. Vet. Res. 2013, 74, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Keir, S.T.; Maris, J.M.; Lock, R.; Kolb, E.A.; Gorlick, R.; Carol, H.; Morton, C.L.; Reynolds, C.P.; Kang, M.H.; Watkins, A.; et al. Initial testing (stage 1) of the multi-targeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr. Blood Cancer 2010, 55, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Pignochino, Y.; Grignani, G.; Cavalloni, G.; Motta, M.; Tapparo, M.; Bruno, S.; Bottos, A.; Gammaitoni, L.; Migliardi, G.; Camussi, G.; et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer 2009, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Cappuzzo, F.; Mazzucchelli, L.; Frattini, M. HER2 in solid tumors: More than 10 years under the microscope; where are we now? Future Oncol. 2014, 10, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R.; Huvos, A.G.; Heller, G.; Aledo, A.; Beardsley, G.P.; Healey, J.H.; Meyers, P.A. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J. Clin. Oncol. 1999, 17, 2781. [Google Scholar] [PubMed]
- Scotlandi, K.; Manara, M.C.; Hattinger, C.M.; Benini, S.; Perdichizzi, S.; Pasello, M.; Bacci, G.; Zanella, L.; Bertoni, F.; Picci, P.; et al. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing’s sarcoma. Eur. J. Cancer 2005, 41, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, S.E.; Geisinger, K.R.; King, T.S.; Sciarrotta, J.; Ward, W.G.; Gold, S.H.; Bos, G.D. Clinicopathologic analysis of HER-2/neu immunoexpression among various histologic subtypes and grades of osteosarcoma. Mod. Pathol. 2001, 14, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.G.; Giordano, T.J.; Sanders, D.; Biermann, J.S.; Baker, L. Absence of HER2/neu gene expression in osteosarcoma and skeletal Ewing’s sarcoma. Clin. Cancer Res. 2002, 8, 788–793. [Google Scholar] [PubMed]
- Gill, J.; Ahluwalia, M.K.; Geller, D.; Gorlick, R. New targets and approaches in osteosarcoma. Pharmacol. Ther. 2013, 137, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Deng, Z.; Zhang, Y.; Yan, L.; Cai, L.; Lei, J.; Xie, Y. The prognostic significance of Src and p-Src expression in patients with osteosarcoma. Med. Sci. Monit. 2015, 21, 638–645. [Google Scholar] [PubMed]
- Akiyama, T.; Dass, C.R.; Choong, P.F.M. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol. Cancer Ther. 2008, 7, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Zhang, W.; Gorlick, R.; Kolb, E.A. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin. Cancer Res. 2009, 15, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montero, C.M.; Wygant, J.N.; McIntyre, B.W. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur. J. Cancer 2006, 42, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.M.; Agrawal, S.; Burris, H.; Rosen, L.; Dhillon, N.; Hong, D.; Blackwood-Chirchir, A.; Luo, F.R.; Sy, O.; Kaul, S.; et al. A Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer 2010, 116, 1582–1591. [Google Scholar] [CrossRef]
- Schuetze, S.; Wathen, K.; Choy, E.; Samuels, B.L.; Ganjoo, K.N.; Staddon, A.P.; von Mehren, M.; Chow, W.A.; Trent, J.C.; Baker, L.H. Results of a Sarcoma Alliance for Research through Collaboration (SARC) phase II trial of dasatinib in previously treated, high-grade, advanced sarcoma. ASCO Meet. Abstr. 2010, 28, 10009. [Google Scholar]
- Baselga, J.; Cervantes, A.; Martinelli, E.; Chirivella, I.; Hoekman, K.; Hurwitz, H.I.; Jodrell, D.I.; Hamberg, P.; Casado, E.; Elvin, P.; et al. Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin. Cancer Res. 2010, 16, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, Y.; Onozawa, Y.; Kurata, T.; Yasui, H.; Goto, I.; Yamazaki, K.; Machida, N.; Watanabe, J.; Shimada, H.; Shi, X.; et al. First report of the safety, tolerability, and pharmacokinetics of the Src kinase inhibitor saracatinib (AZD0530) in Japanese patients with advanced solid tumours. Investig. New Drugs 2013, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Zhao, L.; Chugh, R.; Thomas, D.G.; Lucas, D.R.; Metko, G.; Zalupski, M.M.; Baker, L.H. Results of a phase II study of sirolimus and cyclophosphamide in patients with advanced sarcoma. Eur. J. Cancer 2012, 48, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Laningham, F.; Wu, J.; O’Shaughnessy, M.A.; Molina, K.; Broniscer, A.; Spunt, S.L.; Luckett, I.; Stewart, C.F.; Houghton, P.J.; et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J. Clin. Oncol. 2007, 25, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Quek, R.; Wang, Q.; Morgan, J.A.; Shapiro, G.I.; Butrynski, J.E.; Ramaiya, N.; Huftalen, T.; Jederlinic, N.; Manola, J.; Wagner, A.J.; et al. Combination mTOR and IGF-1R inhibition: Phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin. Cancer Res. 2011, 17, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Egerer, G.; Ferrari, S.; Rassam, H.; Boecker, U.; Bui-Nguyen, B. A phase II trial of second-line pemetrexed in adults with advanced/metastatic osteosarcoma. Eur. J. Cancer 2012, 48, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Warwick, A.B.; Malempati, S.; Krailo, M.; Melemed, A.; Gorlick, R.; Ames, M.M.; Safgren, S.L.; Adamson, P.C.; Blaney, S.M. Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: A Children’s Oncology Group study. Pediatr. Blood Cancer 2013, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Ory, B.; Moriceau, G.; Redini, F.; Heymann, D. mTOR inhibitors (rapamycin and its derivatives) and nitrogen containing bisphosphonates: Bi-functional compounds for the treatment of bone tumours. Curr. Med. Chem. 2007, 14, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. A Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA. 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Deng, Z.; Zhu, Y.; Long, H.; Zhang, S.; Zhao, J. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med. Oncol. 2010, 27, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Zheng, X.F.S. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol. Sin. 2015, 36, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Morton, C.L.; Kolb, E.A.; Gorlick, R.; Lock, R.; Carol, H.; Reynolds, C.P.; Maris, J.M.; Keir, S.T.; Billups, C.A.; et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr. Blood Cancer 2008, 50, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Morton, C.L.; Gorlick, R.; Lock, R.B.; Carol, H.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Keir, S.T.; Kolb, E.A.; et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol. Cancer Ther. 2010, 9, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, E.D.; Versleijen-Jonkers, Y.M.; Roeffen, M.H.; Franssen, G.M.; Houghton, P.J.; Oyen, W.J.; Boerman, O.C.; van der Graaf, W.T. Abstract 2761: Temsirolimus is effective as a single agent and in combination with cisplatin or bevacizumab in preclinical osteosarcoma models. Cancer Res. 2014, 73, 2761. [Google Scholar] [CrossRef]
- Chawla, S.P.; Staddon, A.P.; Baker, L.H.; Schuetze, S.M.; Tolcher, A.W.; D’Amato, G.Z.; Blay, J.-Y.; Mita, M.M.; Sankhala, K.K.; Berk, L.; et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J. Clin. Oncol. 2012, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Chawla, S.P.; Ray-Coquard, I.; Le Cesne, A.; Staddon, A.P.; Milhem, M.M.; Penel, N.; Riedel, R.F.; Bui-Nguyen, B.; Cranmer, L.D.; et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J. Clin. Oncol. 2013, 31, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Gorlick, R.; Kolb, E.A.; Lock, R.; Carol, H.; Morton, C.L.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Phelps, D.; et al. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr. Blood Cancer 2012, 58, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gobin, B.; Battaglia, S.; Lanel, R.; Chesneau, J.; Amiaud, J.; Rédini, F.; Ory, B.; Heymann, D. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014, 344, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.H.A.; Lin, W.-H.; Hsu, J.T.-A.; Hour, T.-C.; Yeh, T.-K.; Ko, S.; Lien, T.-W.; Coumar, M.S.; Liu, J.-F.; Lai, W.-Y.; et al. BPR1K653, a novel Aurora kinase inhibitor, exhibits potent anti-proliferative activity in MDR1 (P-gp170)-mediated multidrug-resistant cancer cells. PLoS ONE 2011, 6, e23485. [Google Scholar] [CrossRef] [PubMed]
- Bayani, J.; Zielenska, M.; Pandita, A.; Al-Romaih, K.; Karaskova, J.; Harrison, K.; Bridge, J.A.; Sorensen, P.; Thorner, P.; Squire, J.A. Spectral karyotyping identifies recurrent complex rearrangements of chromosomes 8, 17, and 20 in osteosarcomas. Genes Chromosomes Cancer 2003, 36, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, J.; Yang, H.; Ge, Z.; Wang, Q.; Zhang, L.; Wu, C.; Wang, J. Silencing of Aurora kinase A by RNA interference inhibits tumor growth in human osteosarcoma cells by inducing apoptosis and G2/M cell cycle arrest. Oncol. Rep. 2014, 31, 1249–1254. [Google Scholar] [PubMed]
- Maris, J.M.; Morton, C.L.; Gorlick, R.; Kolb, E.A.; Lock, R.; Carol, H.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Wu, J.; et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr. Blood Cancer 2010, 55, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.-K.; Wang, Z.-L.; Pan, S.-T.; Ding, H.-Q.; Au, G.H.T.; He, Z.; Zhou, Z.-W.; Xiao, G.; Yang, Y.-X.; Zhang, X.; et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des. Dev. Ther. 2015, 9, 1555–1584. [Google Scholar]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Le Goff, B.; Berreur, M.; Riet, A.; Moreau, A.; Blanchard, F.; Chevalier, C.; Guisle-Marsollier, I.; Léger, J.; Guicheux, J.; et al. Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. J. Pathol. 2007, 211, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Beristain, A.G.; Narala, S.R.; Di Grappa, M.A.; Khokha, R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J. Cell Sci. 2012, 125, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Jung, J.S.; Kim, D.H.; Lim, J.S.; Kim, M.S.; Kong, C.B.; Song, W.S.; Cho, W.H.; Jeon, D.G.; Lee, S.Y.; et al. RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr. Blood Cancer 2011, 56, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Richard, P.; Wittrant, Y.; Battaglia, S.; Pilet, P.; Trichet, V.; Blanchard, F.; Gouin, F.; Pitard, B.; Heymann, D.; et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: Blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007, 67, 7308–7318. [Google Scholar] [CrossRef] [PubMed]
- Gobin, B.; Baud’Huin, M.; Isidor, B.; Heymann, D.; Heymann, M.-F. mAbs targeting RANKL in bone metastasis treatment. In Monoclonal Antibodies in Oncology; Future Medicine Ltd.: London, UK, 2013; pp. 42–53. [Google Scholar]
- Heymann, D. Anti-RANKL therapy for bone tumours: Basic, pre-clinical and clinical evidences. J. Bone Oncol. 2012, 1, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopeck, A.T.; Lipton, A.; Body, J.-J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kroemer, G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012, 1, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shi, Y.; Li, H.; Yang, M.; Liu, G. MicroRNA-144 acts as a tumor suppressor by targeting Rho-associated coiled-coil containing protein kinase 1 in osteosarcoma cells. Mol. Med. Rep. 2015, 12, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Wei, M. MicroRNA-144 suppresses osteosarcoma growth and metastasis by targeting ROCK1 and ROCK. Oncotarget 2015, 6, 10297–10308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, G.; Shen, F.; Kang, Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014, 35, 4859–4865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Liu, J.; Wu, Y.; Zhu, Q. MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma cell lines possibly by targeting Sox. Int. J. Oncol. 2015, 47, 1672–1684. [Google Scholar] [PubMed]
- Salah, Z.; Arafeh, R.; Maximov, V. miR-27a and miR-27a* contribute to metastatic properties of osteosarcoma cells. Oncotarget 2015, 6, 4920. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, H.; Cai, H.; Wu, H. Diagnostic and prognostic potentials of microRNA-27a in osteosarcoma. Biomed. Pharmacother. 2015, 71, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Z.; Wu, S.; Zang, X. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J. Exp. Clin. Cancer Res. 2014, 33, 12. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R.; Sowers, R.; Wenzel, B.D.; Richardson, C.; Meyers, P.A.; Healey, J.H.; Levy, A.S. Impairment of methotrexate transport is common in osteosarcoma tumor samples. Sarcoma 2011, 2011, 834170. [Google Scholar]
- Trippett, T.; Meyers, P.; Gorlick, R.; Steinherz, P. High dose trimetrexate with leucovorin protection in recurrent childhood malignancies: A Phase II trial. Proc. Am. Soc. Clin. Oncol. 1999, 18, 231a. [Google Scholar]
- Rollins, K.D.; Lindley, C. Pemetrexed: A multitargeted antifolate. Clin. Ther. 2005, 27, 1343–1382. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A. Pemetrexed (Alimta): A novel multitargeted antifolate agent. Expert Rev. Anticancer Ther. 2003, 3, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, N.; Walters, D.; Fuchs, B. Pemetrexed, a multitargeted antifolate drug, demonstrates lower efficacy in comparison to methotrexate against osteosarcoma cell lines. Pediatr. Blood Cancer 2008, 50, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.J.; Bell, M.D.; Mackinson, C.; Gupta, R.; Meyers, P.A.; Gorlick, R. Phase Ib/IIa study of sustained release lipid inhalation targeting cisplatin by inhalation in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. ASCO Meet. Abstr. 2007, 25, 9525. [Google Scholar]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wan, M. Methylene diphosphonate-conjugated adriamycin liposomes: Preparation, characteristics, and targeted therapy for osteosarcomas in vitro and in vivo. Biomed. Microdevices 2012, 14, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Muggia, F.M.; Carmichael, J.; Winer, E.P.; Jahanzeb, M.; Venook, A.P.; Skubitz, K.M.; Rivera, E.; Sparano, J.A.; Dibella, N.J.; et al. Efficacy and safety of liposomal anthracyclines in Phase I/II clinical trials. Semin. Oncol. 2004, 31, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. DOXO-EMCH (INNO-206): The first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin. Investig. Drugs 2007, 16, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.; Häring, B.; Medinger, M.; Drevs, J.; Steinbild, S.; Kratz, F.; Mross, K. Phase I and pharmacokinetic study of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin. Clin. Cancer Res. 2007, 13, 4858–4866. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Natale, R.B.; Wolin, E.M.; Laabs, B.; Dinh, H.; Wieland, S.; Levitt, D.J.; Mita, A.C. Pharmacokinetic study of aldoxorubicin in patients with solid tumors. Investig. New Drugs 2015, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Marrano, P.; Kumar, S.; Leadley, M.; Elias, E.; Thorner, P.; Baruchel, S. Nab-Paclitaxel is an active drug in preclinical model of pediatric solid tumors. Clin. Cancer Res. 2013, 19, 5972–5983. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, X.; Zhang, Q.; Hao, L.; Ding, Y.; Xu, H. The efficacy of abraxane on osteosarcoma xenografts in nude mice and expression of secreted protein, acidic and rich in cysteine. Am. J. Med. Sci. 2012, 344, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Yin, H.; Eaves, D.; Currier, M.; Cripe, T.P. Preclinical evaluation of nanoparticle albumin-bound paclitaxel for treatment of pediatric bone sarcoma. Pediatr Blood Cancer 2014, 61, 2096–2098. [Google Scholar] [CrossRef] [PubMed]
| Target | Class | Therapy | Mechanism | Clinical Trial | Outcome | Reference |
|---|---|---|---|---|---|---|
| IGF-1R | Anti- IGF-R antibodies | Cixutumumab | Activation of (PI3K/Akt ) and MAPK | Phase I/II | PD | [37] |
| Phase II | No OR | [38] | ||||
| Phase II | MPFS at 6 weeks | [39] | ||||
| Phase II | MPFS at 6 weeks | [40] | ||||
| Phase II | MPFS at 21.4 weeks | [41] | ||||
| Human mAb SCH 717454 (robatumumab) | Inhibits IGF-R binding and signaling | Phase I/IB (Terminated) | ‒ | NCT00960063 * | ||
| Phase II (Terminated) | ‒ | NCT00617890 * | ||||
| IGF ligand-neutralizing antibodies | BI 836845 mAb | Neutralizes IGF ligand | Phase I | ‒ | NCT01403974 * | |
| Phase I | ‒ | NCT02145741 * | ||||
| Phase I | ‒ | NCT01317420 * | ||||
| Small-molecule TKI’s | BMS-754807 | ATP-competitive inhibitor of IGF | Phase 1I | ‒ | NCT00898716 * | |
| Phase III | ‒ | NCT00134030 * | ||||
| PDGFR | Small-molecule TKI’s | Imatinib mesylate | PDFGR, c-KIT | Phase II | no OR | [42] |
| Phase II | ‒ | NCT00030667 * | ||||
| Phase II | ‒ | NCT00031915 * | ||||
| Multi-targeted RTK inhibitors | Sunitinib | PDGFR, FLT3, RET, KIT and VEGFR inhibitor | Phase I | SD in 1(2) | [43] | |
| VEGF/VEGFR | Anti-VEGF antibodies | Bevacizumab | VEGF inhibitor | Phase I | no CPR | [44] |
| Phase II | NCT00458731 * | |||||
| Small-molecule TKI’s | Sorafenib | PDGFR, FLT3, RET, c-KIT, VEGFR inhibitor | Phase II | 45% PFS at 6 months | [45] | |
| Phase I | ‒ | NCT00665990 * | ||||
| Phase I | ‒ | NCT01518413 * | ||||
| HER-2 | Anti-HER-2 antibody | Trastuzumab | HER-2 inhibitor | Phase II | NSD | [46] |
| Phase II | ‒ | NCT00005033 * |
| Target | Class | Therapy | Mechanism | Clinical Trial | Outcome | Reference |
|---|---|---|---|---|---|---|
| Src | small-molecule TKI’s (Multi targeted) | Dasatinib | PDGF/PDGFR, SRC, BCR-ABL inhibitor | Phase I | SD in 1(1) | [87] |
| Phase II | SD in 5(45) | [88] | ||||
| Phase II | ‒ | NCT00464620 * | ||||
| Phase I/II | ‒ | NCT00788125 * | ||||
| Dual-inhibitor | Saracatinib | Src and Abl specific inhibitor | Phase II | ‒ | NCT00752206 * | |
| mTOR | 1st generation of mTOR inhibitors | Sirolimus | Inhibit mTORC1 by binding toFKBP-12 | Phase II | No CPR | [91] |
| Everolimus | Phase I | SD in 1(2), no OR | [92] | |||
| ‒ | Phase I | SD in 3(3) | [93] | |||
| ‒ | Phase II | ‒ | NCT01216826 * | |||
| Temsirolimus | ‒ | Phase II | MPFS at 21.4 weeks | [41] | ||
| ‒ | Phase II | MPFS at 6 weeks | [40] | |||
| ‒ | Phase II | ‒ | NCT01614795 * | |||
| 2nd generation of dual mTOR inhibitors | AZD8055 | mTOR1, mTOR2 inhibitor | Phase I | ‒ | NCT00731263 * | |
| Phase I withdrawn | ‒ | NCT01194193 * | ||||
| Aurora kinase A | 2nd generation aurora kinase inhibitors | MLN8237 (alisertib) | AURK-Ainhibitor via ATP binding | Phase I | ‒ | NCT02214147 * |
| Phase I | ‒ | NCT01898078 * | ||||
| Phase II | ‒ | NCT01154816 * | ||||
| Phase I | ‒ | NCT02444884 * | ||||
| Folate | Multitargeted antifolate | Pemetrexed | folate-dependent enzymes and DNA synthesis enzymes inhibitor | Phase II | CPR in 1 | [94] |
| SD in 5 | ||||||
| PD in 22 | ||||||
| MPFS at 1.4 months | ||||||
| Phase II | no CPR | [95] | ||||
| Phase II | ‒ | NCT00003776 * | ||||
| Phase II | ‒ | NCT00002738 * | ||||
| Phase I | ‒ | NCT00119301 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, A.B.; Li, F.; Li, M.; He, B.; He, X.; Chen, G.; Guo, B.; Li, D.; Jiang, F.; Dang, L.; et al. Present Advances and Future Perspectives of Molecular Targeted Therapy for Osteosarcoma. Int. J. Mol. Sci. 2016, 17, 506. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040506
Shaikh AB, Li F, Li M, He B, He X, Chen G, Guo B, Li D, Jiang F, Dang L, et al. Present Advances and Future Perspectives of Molecular Targeted Therapy for Osteosarcoma. International Journal of Molecular Sciences. 2016; 17(4):506. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040506
Chicago/Turabian StyleShaikh, Atik Badshah, Fangfei Li, Min Li, Bing He, Xiaojuan He, Guofen Chen, Baosheng Guo, Defang Li, Feng Jiang, Lei Dang, and et al. 2016. "Present Advances and Future Perspectives of Molecular Targeted Therapy for Osteosarcoma" International Journal of Molecular Sciences 17, no. 4: 506. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040506