Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota
Abstract
:1. Introduction
2. Oral Ingestion of Nanoparticles
2.1. Estimated Amounts of Daily Intake
2.2. Changes of Nanoparticle Properties in the Gastrointestinal Tract
3. Antimicrobial Activity of Nanoparticles
4. Effects of Nanoparticles on Prokaryotic and Eukaryotic Cells
4.1. Mechanisms of Ag Nanoparticle (NP) Action
4.2. Mechanisms of ZnO NP Action
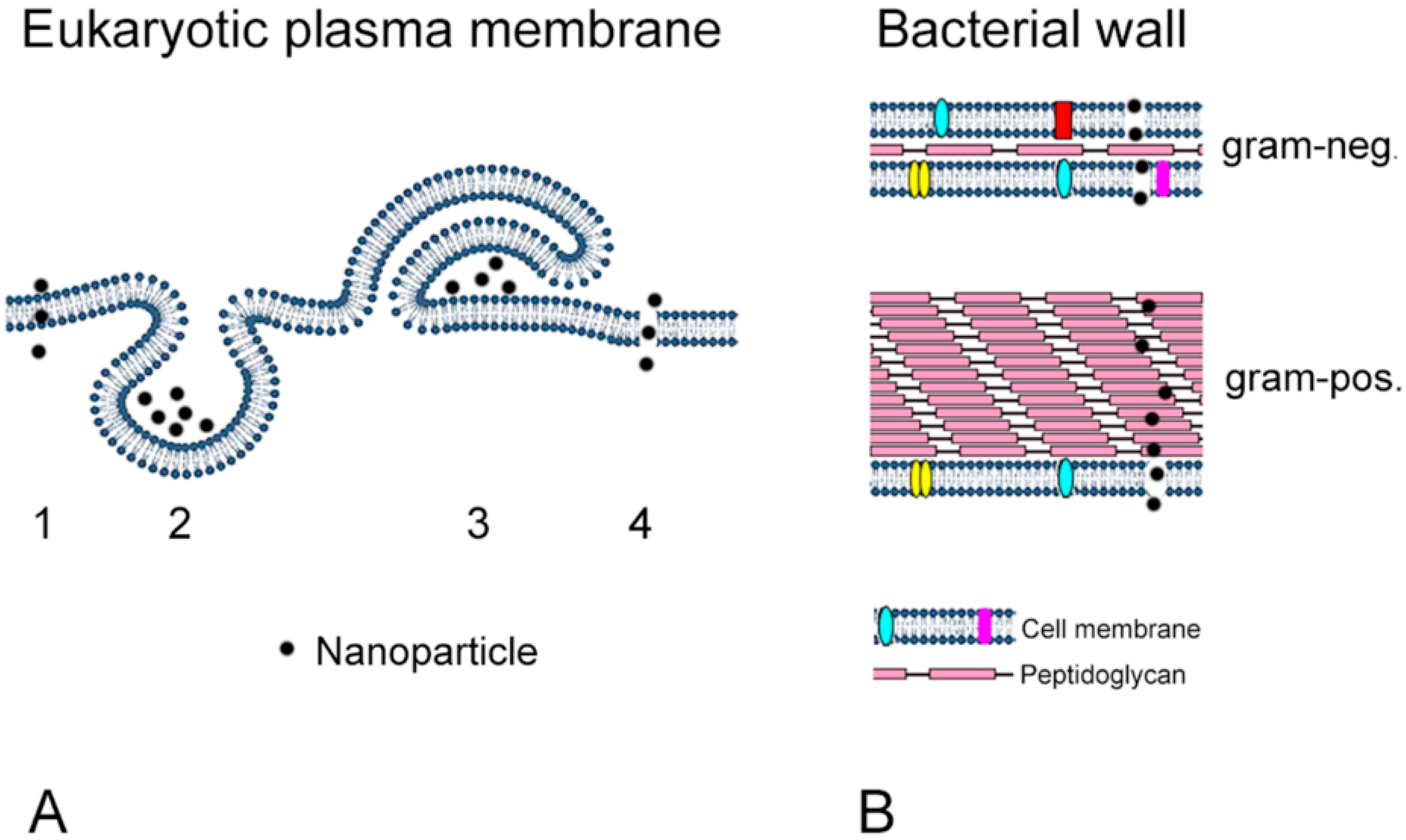
5. Comparison between Intestinal Cells and Bacterial Toxicity
5.1. Antimicrobial Effects
5.2. Adverse Effects of Nanoparticles on Intestinal Cells (Enterocytes)
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Draganov, P.V. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J. Gastroenterol. 2009, 15, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Damman, C.J.; Miller, S.I.; Surawicz, C.M.; Zisman, T.L. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Ellingrod, V.L. The microbiome in mental health: Potential contribution of gut microbiota in disease and pharmacotherapy management. Pharmacotherapy 2015, 35, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Isolauri, E.; Salminen, S. Gut microbiota: A source of novel tools to reduce the risk of human disease? Pediatr. Res. 2015, 77, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Flavell, R.A. Immune-microbiota interactions in health and disease. Clin. Immunol. 2015, 159, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Nell, S.; Suerbaum, S.; Josenhans, C. The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat. Rev. Microbiol. 2010, 8, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Safdar, N. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. J. Hosp. Med. 2016, 11, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Marcus, I.; Guysi, R.; Walker, S. Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ. Eng. Sci. 2015, 32, 602–612. [Google Scholar] [CrossRef]
- Hadrup, N.; Loeschner, K.; Bergstrom, A.; Wilcks, A.; Gao, X.; Vogel, U.; Frandsen, H.L.; Larsen, E.H.; Lam, H.R.; Mortensen, A. Subacute oral toxicity investigation of nanoparticulate and ionic silver in rats. Arch. Toxicol. 2012, 86, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Sawosz, E.; Binek, M.; Grodzik, M.; Zielinska, M.; Sysa, P.; Szmidt, M.; Niemiec, T.; Chwalibog, A. Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. Arch. Anim. Nutr. 2007, 61, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.; Herrer, R.; Casallas, M.; Abecia, L.; Ducha, J. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim. Feed. Sci. Technol. 2009, 150, 259–269. [Google Scholar] [CrossRef]
- Das, P.; McDonald, J.; Petrof, E.; Allen-Vercoe, E.; Walker, V. Nanosilver-mediated change in human intestinal microbiota. J. Nanomed. Nanotechnol. 2014, 5, 1–10. [Google Scholar]
- Brooks, J.P.; Edwards, D.J.; Harwich, M.D., Jr.; Rivera, M.C.; Fettweis, J.M.; Serrano, M.G.; Reris, R.A.; Sheth, N.U.; Huang, B.; Girerd, P.; et al. The truth about metagenomics: Quantifying and counteracting bias in 16S rRNA studies. BMC Microbiol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Ben-Slama, I.; Mrad, I.; Rihane, N.; Mir, L.; Sakly, M.; Amara, S. Sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J. Nanomed. Nanotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.; Liang, S.; Li, W.; Wu, X.; Wang, L.; Jin, F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 2015, 6, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Ivask, A. Mapping the dawn of nanoecotoxicological research. Acc. Chem. Res. 2013, 46, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Von Goetz, N.; Fabricius, L.; Glaus, R.; Weitbrecht, V.; Gunther, D.; Hungerbuhler, K. Migration of silver from commercial plastic food containers and implications for consumer exposure assessment. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2013, 30, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, S.; Bing, X.; Gao, C.; Wang, T.; Yuan, B. Nanosilver migrated into food-simulating solutions from commercially available food fresh containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Processing Aids Processing Aids EFSA Panel on Food Additives, Flavourings and Materials in Contact with Foods (AFC). Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (afc) related to the 12th list of substances for food contact materials. Avaliable online: http://www.efsa.europa.eu/de/efsajournal/pub/395 (accessed on 20 March 2016).
- Echegoyen, Y.; Nerin, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Silver in drinking-water. Background document for preparation of who guidelines for drinking-water quality. WHO/SDE/WSH/03.04/14. 2003. Avaliable online: http://www.who.int/entity/water_sanitation_health/dwq/chemicals/silver.pdf (accessed on 20 March 2016).
- Dekkers, S.; Krystek, P.; Peters, R.J.; Lankveld, D.P.; Bokkers, B.G.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Toxicity, Ecotoxicity and the Environment. Opinion on the results of the Risk Assessment of: Zinc metal (CAS No. 7440-66-6), Zinc chloride (CAS No. 7646-85-7), Zinc sulphate (CAS No. 7733-02-0), Zinc distearate (CAS No. 557-05-1, 9105-01-3), Zinc phosphate (CAS No. 779-90-0), Zinc oxide (CAS No. 1314-13-2) Human Health Part. In Proceedings of the 39th plenary meeting, Brussels, Belgian, 10 September 2003.
- Hansen, S.F.; Michelson, E.S.; Kamper, A.; Borling, P.; Stuer-Lauridsen, F.; Baun, A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology 2008, 17, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Thompson, R.P.; Commisso, J.; Keen, C.L.; Powell, J.J. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. Analyst 2000, 125, 2339–2343. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Hutchinson, C.; Volkert, S.; Greenfield, S.M.; Catterall, A.; Thompson, R.P.; Powell, J.J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br. J. Nutr. 2004, 92, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Irving, K.; Gregory, J. The National Diet & Nutrition Survey: Adults Aged 19 to 64 Years. Vitamin and Mineral Intake and Urinary Analytes; Controller of Her Majesty’s Stationery Office (HMSO): Norwich, UK, 2003. [Google Scholar]
- Ferreira-Pego, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rincker, M.J.; Hill, G.M.; Link, J.E.; Meyer, A.M.; Rowntree, J.E. Effects of dietary zinc and iron supplementation on mineral excretion, body composition, and mineral status of nursery pigs. J. Anim. Sci. 2005, 83, 2762–2774. [Google Scholar] [PubMed]
- Croteau, M.N.; Dybowska, A.D.; Luoma, S.N.; Valsami-Jones, E. A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures. Nanotoxicology 2011, 5, 79–90. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, C.A.; Ogunniyi, A.D.; Valkov, E.; Lawrence, M.C.; Kobe, B.; McEwan, A.G.; Paton, J.C. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 2011, 7, e1002357. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Mucus as barrier for drug delivery by nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.; Basit, A. The Role of Polymers in Solid Dosage Forms. In Polymers in Drug Delivery; Uchegbu, I.F., Schätzlein, A.G.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 35–48. [Google Scholar]
- Jani, P.; McCarthy, D.; Florence, A. Titanium dioxide (rutile) particles uptake from the rat GI tract and translocation to the systemic organs after oral administration. Int. J. Pharm. 1994, 105, 157–168. [Google Scholar] [CrossRef]
- Tay, C.; Setyawati, M.; Xie, J.; Parak, W.; Leong, D. Back to basics: Exploiting the innate physico-chemical characteristics of nanomaterials for biomedical applications. Adv. Funct. Mater. 2014, 24, 5936–5955. [Google Scholar] [CrossRef]
- Bellmann, S.; Carlander, D.; Fasano, A.; Momcilovic, D.; Scimeca, J.A.; Waldman, W.J.; Gombau, L.; Tsytsikova, L.; Canady, R.; Pereira, D.I.; et al. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Fokkink, R.; Peters, R.; Tromp, P.; Herrera Rivera, Z.E.; Rietjens, I.M.; Hendriksen, P.J.; Bouwmeester, H. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology 2013, 7, 1198–1210. [Google Scholar] [PubMed]
- Axson, J.; Stark, D.; Bondy, A.; Capracotta, S.; Maynard, A.; Philbert, M.; Bergin, I.; Ault, A. Rapid kinetics of size and pH-dependent dissolution and aggregation of silver nanoparticles in simulated gastric fluid. J. Phys. Chem. C 2015, 119, 20632–20641. [Google Scholar] [CrossRef]
- Mwilu, S.K.; El Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kato, K.; Hidaka, M.; Un, K.; Kawanishi, T.; Okuda, H. Physicochemical properties and in vitro intestinal permeability properties and intestinal cell toxicity of silica particles, performed in simulated gastrointestinal fluids. Biochim. Biophys. Acta 2014, 1840, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Kramer, E.; Oomen, A.G.; Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.; Weigel, S.; et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Ebmeyer, J.; Knappe, P.; Juling, S.; Bohmert, L.; Selve, S.; Niemann, B.; Braeuning, A.; Thunemann, A.F.; Lampen, A. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol. Chem. 2015, 396, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Pereira, D.I.; Faria, N.; Boots, A.W.; Kolling, J.; Forster, I.; Albrecht, C.; Powell, J.J.; Schins, R.P. Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco-2 cells. Nanotoxicology 2013, 7, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, H.; Ramaker, K.; Frey, A. Coating with luminal gut-constituents alters adherence of nanoparticles to intestinal epithelial cells. Beilstein J. Nanotechnol. 2014, 5, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gelover, S.; Gomez, L.A.; Reyes, K.; Teresa Leal, M. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomedicine 2009, 5, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Camporotondi, D.; Foglia, M.; Alvarez, G.; Mebert, A.; Diaz, L.; Coradin, T.; Desimone, M. Antimicrobial properties of silica modified nanoparticles. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 283–290. [Google Scholar]
- The Source for Critical Information and Insight. Specialty Chemicals Update Program: Nanoscale Chemicals and Materials; IHS Inc.: Douglas County, CO, USA, 2010. [Google Scholar]
- Lonhienne, T.G.; Sagulenko, E.; Webb, R.I.; Lee, K.C.; Franke, J.; Devos, D.P.; Nouwens, A.; Carroll, B.J.; Fuerst, J.A. Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc. Natl. Acad. Sci. USA 2010, 107, 12883–12888. [Google Scholar] [CrossRef] [PubMed]
- Saladin, K. Part One Cytology—The Study of Cells. In Human Anatomy; Saladin, K., Ed.; McGraw-Hill Higher Education: New York, NY, USA, 2007; pp. 47–76. [Google Scholar]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Pust, S.; Skotland, T.; van Deurs, B. Clathrin-independent endocytosis: Mechanisms and function. Curr. Opin. Cell Biol. 2011, 23, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.T. Anatase TiO2 nanocomposites for antimicrobial coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.F. A paradigm gets shifty. Nature 1998, 392, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. Curr. Drug Metab. 2013, 14, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef] [PubMed]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Wilson, C.G. The transit of dosage forms through the colon. Int. J. Pharm. 2010, 395, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alsalhi, M.S.; Siddiqui, M.K. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Miethling-Graff, R.; Rumpker, R.; Richter, M.; Verano-Braga, T.; Kjeldsen, F.; Brewer, J.; Hoyland, J.; Rubahn, H.G.; Erdmann, H. Exposure to silver nanoparticles induces size- and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Vitro 2014, 28, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yu, X.; Xu, C.; Li, X.; Li, Z.; Wei, D.; Liu, Y. New toxicity mechanism of silver nanoparticles: Promoting apoptosis and inhibiting proliferation. PLoS ONE 2015, 10, e0122535. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Toolabi, A.; Khanjani, N. Evaluating the toxicity of Zinc Oxide Nanoparticles on the dominant bacteria in the sludge of wastewater treatment facilities. Adv. Environ. Biol. 2013, 7, 812–816. [Google Scholar]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Vandebriel, R.; De Jong, W. A review of mammalian toxicity of ZnO nanoparticles Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar]
- McCracken, C.; Zane, A.; Knight, D.A.; Dutta, P.K.; Waldman, W.J. Minimal intestinal epithelial cell toxicity in response to short- and long-term food-relevant inorganic nanoparticle exposure. Chem. Res. Toxicol. 2013, 26, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Albrecht, C.; Boots, A.; Förster, I.; Schins, R. Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cells. Nanotoxicology 2009, 3, 355–364. [Google Scholar] [CrossRef]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Abbott Chalew, T.E.; Schwab, K.J. Toxicity of commercially available engineered nanoparticles to Caco-2 and SW480 human intestinal epithelial cells. Cell Biol. Toxicol. 2013, 29, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; de Berardis, B.; Bizzarri, L.; Degan, P.; Andreoli, C.; Zijno, A.; de Angelis, I. Physico-chemical characteristics and cyto-genotoxic potential of ZnO and TiO2 nanoparticles on human colon carcinoma cells. J. Phys. Conf. Ser. 2011, 304. [Google Scholar] [CrossRef]
- Kilari, S.; Pullakhandam, R.; Nair, K.M. Zinc inhibits oxidative stress-induced iron signaling and apoptosis in Caco-2 cells. Free Radic. Biol. Med. 2010, 48, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.; Ann, L.; Bakhori, S.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Fan, D.; Gao, J.; Zou, W.; Peng, Z.; Zhao, Z.; Ling, J.; LeGeros, R.Z. Effect of ZnCl2 on plaque growth and biofilm vitality. Arch. Oral Biol. 2012, 57, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gull, I.; Saeed, M.; Shaukat, H.; Aslam, S.M.; Samra, Z.Q.; Athar, A.M. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Blount, Z.D. The unexhausted potential of E. coli. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Zhao, Y.; Huang, J. Metabolic modeling of common Escherichia coli strains in human gut microbiome. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guerin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, J.; Osaka, T.; Park, S. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, J.S.; Infante, H.G.; Stokes, E.; Shaw, A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Ivask, A.; Kakinen, A.; Kurvet, I.; Kahru, A. Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.; Akhter, J.; Hameed, A.; Hasan, M. Antibacterial characterization of silver nanoparticles against E. Coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver nanoparticles in therapeutics: Development of an antimicrobial gel formulation for topical use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.R.; Bailey, F.C.; Elrod-Erickson, M.; Neigh, A.M.; Otter, R.R. Effects of silver nanoparticles on zebrafish (Danio rerio) and Escherichia coli (ATCC 25922): A comparison of toxicity based on total surface area versus mass concentration of particles in a model eukaryotic and prokaryotic system. Environ. Toxicol. Chem. 2012, 31, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Martinez-Castanon, G.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guerin. J. Nanobiotechnol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Durna, N. Biogenic Silver Nanoparticles: Application in Medicines and Textiles. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin, Germany, 2011; pp. 249–268. [Google Scholar]
- El-Kheshen, A.; El-Rab, S. Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Pharm. Chem. 2012, 4, 53–65. [Google Scholar]
- Devi, J.; Bhimba, B. Anticancer activity of silver nanoparticles synthesized by the seaweed Ulva lactuca in vitro. Open Access Sci. Rep. 2012, 1, 242. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]
- Manna, A. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles. In Nano-Antimicrobials; Cioffi, N., Rai, M., Eds.; Springer: Berlin, Germany, 2012; pp. 151–180. [Google Scholar]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Sultan, A.; Azam, A. Synthesis and characterization of the antibacterial potential of ZnO nanoparticles against extended-spectrum beta-lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated from a tertiary care hospital of North India. Appl. Microbiol. Biotechnol. 2012, 94, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Tech. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Baek, Y.W.; An, Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Bazes, A.; Schneider, Y.J. In vitro toxicity assessment of silver nanoparticles in the presence of phenolic compounds—Preventive agents against the harmful effect? Nanotoxicology 2014, 8, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Poortman, J.; Peters, R.J.; Wijma, E.; Kramer, E.; Makama, S.; Puspitaninganindita, K.; Marvin, H.J.; Peijnenburg, A.A.; Hendriksen, P.J. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano 2011, 5, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.H.; Chen, C.F.; Huang, S.; Shih, T.S.; Lai, P.S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N.; Readman, J.A.J.; Readman, J.W.; Lowe, D.W.; Frickers, P.E.; Beesley, A. Lysosomal cytotoxicity of carbon nanoparticles in cells of the molluscan immune system: An in vitro study. Nanotoxicology 2009, 3, 40–45. [Google Scholar] [CrossRef]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Kakinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Don, M.; San, C.; Jeevanandam, J. Antimicrobial properties of nanobiomaterials and the mechanism. In Nanobiomaterials in Antimicrobial Therapy: Applications of Nanobiomaterials; Grumezescu, A., Ed.; Elsevier Inc.: Oxford, UK, 2016; pp. 261–312. [Google Scholar]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Meindl, C.; Pieber, T. Important issues in the cytotoxicity screening of nano-sized materials. EURONanoTox Lett. 2010, 1, 1–6. [Google Scholar]
- Bohmert, L.; Girod, M.; Hansen, U.; Maul, R.; Knappe, P.; Niemann, B.; Weidner, S.M.; Thunemann, A.F.; Lampen, A. Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells. Nanotoxicology 2014, 8, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Roursgaard, M.; Kermanizadeh, A.; Loft, S.; Moller, P. Synergistic effects of zinc oxide nanoparticles and fatty acids on toxicity to Caco-2 cells. Int. J. Toxicol. 2015, 34, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Withington, L. High-throughput epithelial cell culture systems for screening drug intestinal permeability. In Cell Culture Models of Biological Barriers. in Vitro Test Systems for Drug Absorption and Delivery; Lehr, C.-M., Ed.; Taylor & Francis: London, UK, 2002; pp. 94–111. [Google Scholar]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wick, P. Human health risk of ingested nanoparticles that are added as multifunctional agents to paints: An in vitro study. PLoS ONE 2013, 8, e83215. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Forster, I.; Schins, R.P. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, A.; Lanceleur, R.; Mourot, A.; Lavault, M.T.; Casterou, G.; Jarry, G.; Hogeveen, K.; Fessard, V. Toxicity, genotoxicity and proinflammatory effects of amorphous nanosilica in the human intestinal Caco-2 cell line. Toxicol. Vitro 2015, 29, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Bohmert, L.; Niemann, B.; Thunemann, A.F.; Lampen, A. Cytotoxicity of peptide-coated silver nanoparticles on the human intestinal cell line Caco-2. Arch. Toxicol. 2012, 86, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guan, R.; Lyu, F.; Kang, T.; Wu, Y.; Chen, X. In vitro cytotoxicity of silver nanoparticles and zinc oxide nanoparticles to human epithelial colorectal adenocarcinoma (Caco-2) cells. Mutat. Res. 2014, 769, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Aueviriyavit, S.; Phummiratch, D.; Maniratanachote, R. Mechanistic study on the biological effects of silver and gold nanoparticles in Caco-2 cells—Induction of the Nrf2/HO-1 pathway by high concentrations of silver nanoparticles. Toxicol. Lett. 2014, 224, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.J.; Doudrick, K.; Yang, Y.; Westerhoff, P.; Capco, D.G. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol. Toxicol. 2014, 30, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Griesbacher, A.; Pieber, T.R. Cytotoxicity of nanoparticles is influenced by size, proliferation and embryonic origin of the cells used for testing. Nanotoxicology 2012, 6, 424–423. [Google Scholar] [CrossRef] [PubMed]
- Bionumbers. Available online: http://bionumbers.hms.harvard.edu/bionumber.aspx?id=108905 (accessed on 20 March 2016).
- Moos, P.J.; Olszewski, K.; Honeggar, M.; Cassidy, P.; Leachman, S.; Woessner, D.; Cutler, N.S.; Veranth, J.M. Responses of human cells to ZnO nanoparticles: A gene transcription study. Metallomics 2011, 3, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Moos, P.J.; Chung, K.; Woessner, D.; Honeggar, M.; Cutler, N.S.; Veranth, J.M. ZnO particulate matter requires cell contact for toxicity in human colon cancer cells. Chem. Res. Toxicol. 2010, 23, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. CSH Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical transformations of nanosilver in biological environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Reeder, J.; Gibert, P.; Varela, E.; Llopis, M.; Antolin, M.; Guigo, R.; Knight, R.; Guarner, F. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010, 20, 1411–1419. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticle | Size (nm) | Bacterial Strain | Effect | Reference |
|---|---|---|---|---|
| Ag | 3 | E. coli (−) | MIC: 40 µg/mL | [103] |
| S. aureus (+) | 120 µg/mL | |||
| Ag | 8, citrate | E. coli (−) | MIC: 8 µg/mL | [104] |
| Ag | 10, PVP | E. coli (−) | MIC: 10 µg/mL | [100] |
| S. aureus (+) | 5 µg/mL | |||
| Ag | 10.5, PVP | E. coli (−) | EC50: 8.9 mg/L | [105] |
| B. subtilis (+) | 5.2 mg/L | |||
| S. aureus (+) | 16.1 mg/L | |||
| P. aeruginosa (−) | 0.59 mg/L | |||
| Ag | 12.7 | E. coli (−) | MIC: >10 µg/mL | [106] |
| Ag | 13.4 | E. coli (−) | MIC: >0.35 µg/L | [107] |
| S. aureus (+) | >3.56 µg/L | |||
| Ag | 10–15 | E. coli (−) | MIC: 25 µg/mL | [108] |
| S. aureus (+) | 100 µg/mL | |||
| Ag | 16 | E. coli (−) | MIC: 60 mg/L | [109] |
| Ag | 4, <20, biostabilized | E. coli (−) | MIC: 2, 0.5 mg/L | [110] |
| B. subtilis (+) | 6, 2 mg/L | |||
| Ag | 7–20, biostabilized | E. coli (−) | MIC: 6.3 mg/L | [111] |
| B. subtilis (+) | 6.3 mg/L | |||
| P. aeruginosa (−) | 6.3 mg/L | |||
| S. aureus (+) | 12.5 mg/L | |||
| Ag | 20 | E. coli (−) | Inhib: 20 and 23 mm at 10 µg/mL | [112] |
| S. aureus (+) | ||||
| Ag | 21 | E. coli (−) | MIC: 75 µg/mL | [113] |
| Ag | 5, 7, 10, 15, 20, 30, 50, 63, 85, 100, citrate | E. coli (−) | MIC: 20, 20, 30, 30, 40, 50, 80, 90, 90, 110 µg/mL | [102] |
| S. aureus (+) | 70, 70, 80, 100, 90, 100, 130, 160, 180, 200 µg/mL | |||
| Ag | 20, 50, 110, citrate | E. coli (−) | EC50: 25, 79, 175 mg/L | [114] |
| Ag | 26 | E. coli (−) | MIC: 1.69 µg/mL | [115] |
| P. aeruginosa (−) | 3.38 µg/mL | |||
| S. aureus (+) | 3.38 µg/mL | |||
| K. pneumoniae (−) | 6.75 µg/mL | |||
| Ag | 1, 29, 89 | E. coli (−) | MIC: 6.3, 13, 11.8 mg/L 7.5, 16.7, 33.7 mg/L | [116] |
| S. aureus (+) | ||||
| Ag | 30 | E. coli (−) | MIC: 5–10 µg/mL | [117] |
| Ag | 25, >25 | E. coli (−) | MIC: 1.69–13.5 µg/mL, 6.75–54 µg/mL | [118] |
| Ag | 20–60, PVP | E. coli (−) | MIC: 125 µg/mL | [119] |
| Ag | 40–50 | S. aureus (+) | Inhib: 15–25 mm at 39.5 µg/mL | [120] |
| B subtilis (+) | ||||
| K. pneumoniae (−) | ||||
| E. coli (−) | ||||
| Ag | 50 | E. coli (−) | MIC: 0.1 µg/mL | [121] |
| Ag | 55 | E. coli (−) | MIC: 0.25 µg/mL | [122] |
| S. aureus (+) | ||||
| ZnO | 3 | E. coli (−) | MIC: 3.1 mg/mL | [123] |
| S. aureus (+) | 1.5 mg/mL | |||
| ZnO | 8, 11, 13 | E. coli (−) | MIC: >244 mg/L; | [124] |
| S. aureus (+) | 81.41 mg/L | |||
| ZnO | 19 | E. coli (−) | MIC: 500 mg/L | [125] |
| S. aureus (+) | 1000 mg/L | |||
| K. pneumoniae (−) | 500 mg/L | |||
| ZnO | 30 | E. coli (−) | MIC: 0.4 mg/mL | [93] |
| ZnO | 47 | E. coli (−) | MIC: 400 mg/L | [126] |
| ZnO | 50–70 | E. coli (−) | EC50: 115.7 mg/L | [127] |
| B. subtilis (+) | 85.8 mg/L | |||
| S. aureus (+) | >125 mg/L | |||
| ZnO | 70 | E. coli (−) | MIC: 972 mg/L | [128] |
| Nanoparticle | Size (nm) | Intestinal Cells | Effect | Reference |
| Ag | <20 | Caco-2/Raji B | EC50: 40 µg/mL | [129] |
| Ag | 18 | HT-29 | EC50: 25 µg/mL | [130] |
| Ag | 20–30 | Caco-2 | EC50: >100 µg/mL | [89] |
| Ag | 40–50 | HT-29 | EC50 (HT-29): 39.5 µg/mL | [120] |
| Ag | 20, 34, 61, 113 | Caco-2/Raji B | Viab. >80% at 50 µg/mL | [131] |
| Ag | 1–100 | HCT116 | sign. decr. viab.: 50 µg/mL | [132] |
| ZnO | 20–60 | RKO | 30% viab. at 30 µg/mL | [133] |
| ZnO | 26, 62, 90 | Caco-2 | EC50: 15.6, 22.9, 18.6 µg/mL | [134] |
| ZnO | 50–70 | LoVo | sign. viab. decr. at 10 µg/mL | [88] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, E.E.; Fröhlich, E. Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota. Int. J. Mol. Sci. 2016, 17, 509. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040509
Fröhlich EE, Fröhlich E. Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota. International Journal of Molecular Sciences. 2016; 17(4):509. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040509
Chicago/Turabian StyleFröhlich, Esther E., and Eleonore Fröhlich. 2016. "Cytotoxicity of Nanoparticles Contained in Food on Intestinal Cells and the Gut Microbiota" International Journal of Molecular Sciences 17, no. 4: 509. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040509








