Ionone Derivatives from the Mycelium of Phellinus linteus and the Inhibitory Effect on Activated Rat Hepatic Stellate Cells
Abstract
:1. Introduction
2. Results
2.1. Purification and Characterization
2.2. Structural Elucidation of Compounds 1–3
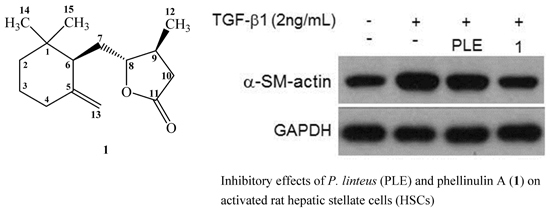
3. Discussion
4. Materials and Methods
4.1. General
4.2. Materials
4.3. Extraction and Isolation
4.3.1. Phellinulin A (1)
4.3.2. Phellinulin B (2)
4.3.3. Phellinulin C (3)
4.4. Determination of the Inhibition Effect on Activated Rat Hepatic Stellate Cells
4.4.1. Cell Culture and MTT Assay
4.4.2. Western Blot Analysis
4.4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Micco, L.; Conti, F.; Andreone, P.; Bernardi, M. Alcohol and viral hepatitis: A mini-review. Dig. Liver Dis. 2009, 41, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Abboud, G.; Kaplowitz, N. Drug-induced liver injury. Drug Saf. 2007, 30, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.; Hellerbrand, C. Obesity and fatty liver are ’grease’ for the machinery of hepatic fibrosis. Dig. Dis. 2011, 29, 377–383. [Google Scholar] [PubMed]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Liao, Z.X.; Ping, J.; Xu, D.; Wang, H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008, 43, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Aldo, D.; Giovanni, A.; Domenico, M.; Francesco, P.; Antonella, A. New therapeutic approaches to liver fibrosis: A practicable route? Curr. Med. Chem. 2008, 15, 1628–1644. [Google Scholar] [PubMed]
- Holt, A.P.; Salmon, M.; Buckley, C.D.; Adams, D.H. Immune interactions in hepatic fibrosis. Clin. Liver Dis. 2008, 12, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Forner, J.; Llovet, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Elsharkawy, A.M.; Oakley, F.; Mann, D.A. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis 2005, 10, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Ghiassi-Nejad, Z.; Friedman, S.L. Advances in antifibrotic therapy. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Gores, G.J. Apoptosis as a mechanism for liver disease progression. Semin. Liver Dis. 2010, 30, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.F.; Wu, Y.T.; Tsai, T.H. Biological analysis of herbal medicines used for the treatment of liver diseases. Biomed. Chromatogr. 2011, 25, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Kwon, H.K.; Kim, J.E.; Rho, J.; Im, S.H. Immunomodulatory effect of water soluble extract separated from mycelium of Phellinus linteus on experimental atopic dermatitis. BMC Complement Altern. Med. 2012, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsugo, S.; Uzuka, Y.; Matsuo, S.; Kawagishi, H. Fractionation and anti-tumor activity of the mycelia of liquid-cultured Phellinus linteus. Biosci. Biotechnol. Biochem. 2004, 68, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Lee, C.W.; Jeon, Y.J.; Hong, N.D.; Yoo, I.D.; Yang, K.H.; Kim, H.M. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology 1999, 41, 157–164. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, Y.S.; Kim, S.K.; Kim, B.C.; Lim, C.J.; Park, E.H. Anti-inflammatory and related pharmacological activities of the n-BuOH subfraction of mushroom Phellinus linteus. J. Ethnopharmacol. 2004, 93, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O.; Yamada, K.; Cho, B.G.; Jeon, T.; Hwang, S.G.; Park, T.; Kang, S.A.; Park, D.K. Comparative study on the modulation of IgE and cytokine production by Phellinus linteus grown on germinated brown Rice, Phellinus Linteus and germinated brown rice in murine splenocytes. Biosci. Biotechnol. Biochem. 2004, 68, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Shibata, T.; Itoh, T.; Suzuki, T.; Tanaka, H.; Nakamura, T.; Akiyama, Y.; Kawagishi, H.; Nagai, H. Inhibition of IgE-dependent mouse triphasic cutaneous reaction by a boiling water fraction separated from mycelium of Phellinus linteus. Evid. Based Complement. Altern. Med. 2005, 2, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kwon, Y.J.; Sohn, M.J.; Seok, S.J.; Kim, W.G. Phellinstatin, a new inhibitor of enoyl-ACP reductase produced by the medicinal fungus Phellinus linteus. Bioorg. Med. Chem. Lett. 2011, 21, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.J.; Mo, S.Y.; Li, S.; Yang, Y.C.; Shi, J.G. Phelligridimer A, a highly oxygenated and unsaturated 26-membered macrocyclic metabolite with antioxidant activity from the fungus Phellinus Igniarius. Org. Lett. 2005, 7, 4733–4736. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Seok, S.J.; Kim, W.K.; Yun, B.S. Hispidin derivatives from the mushroom Inonotus xeranticus and their antioxidant activity. J. Nat. Prod. 2006, 69, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Wang, S.; Zhou, G.; Yang, Y.; Li, Y.; Chen, X.; Shi, J. Phelligridins C–F: Cytotoxic pyrano[4,3-c][2]benzopyran-1,6-dione and furo[3,2-c]pyran-4-one derivatives from the fungus Phellinus igniarius. J. Nat. Prod. 2004, 67, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Yun, B.S. Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus. Bioorg. Med. Chem. 2007, 15, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Janardhanan, K.K. Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J. Ethnopharmacol. 2002, 81, 387–391. [Google Scholar] [CrossRef]
- Jeon, T.I.; Hwang, S.G.; Lim, B.O.; Park, D.K. Extracts of Phellinus linteus grown on germinated brown rice suppress liver damage induced by carbon tetrachloride in rats. Biotechnol. Lett. 2003, 25, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, H.S.; Lee, S.; Cho, J.; Ze, K.; Sung, J.; Kim, Y.C. Mycelial culture of Phellinus linteus protects primary cultured rat hepatocytes against hepatotoxins. J. Ethnopharmacol. 2004, 95, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Peng, W.H.; Sheu, M.J.; Huang, G.J.; Tseng, M.C.; Lai, M.T.; Ho, Y.L.; Chang, Y.S. Hepatoprotective and antioxidant effects of ethanol extract from Phellinus merrillii on carbon tetrachloride-induced liver damage. Am. J. Chin. Med. 2007, 35, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, G.; Park, H.J.; Jiang, P.P.; Sit, W.H.; van Griensven, L.J.; Wan, J.M. Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: A proteomics analysis. Chin. Med. 2012, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Chiu, Y.T.; Lee, C.Y.; Lin, Y.L.; Huang, Y.T. Increases in fibrosis-related gene transcripts in livers of dimethylnitrosamine-intoxicated rats. J. Biomed. Sci. 2004, 11, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.C.; Wang, P.W.; Pan, T.L.; Bazylak, G.; Leu, Y.L. Proteomic screening of antioxidant effects exhibited by radix Salvia miltiorrhiza aqueous extract in cultured rat aortic smooth muscle cells under homocysteine treatment. J. Ethnopharmacol. 2009, 124, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Hung, Y.C.; Wang, P.W.; Chen, S.T.; Hsu, T.K.; Sintupisut, N.; Cheng, C.S.; Lyu, P.C. Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics 2010, 10, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Gallin, J.I. Neutrophils in human diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ni, M.M.; Li, X.; Li, X.F.; Meng, X.M.; Huang, C.; Li, J. NLRC5 regulates TGF-β1-induced proliferation and activation of hepatic stellate cells during hepatic fibrosis. Int. J. Biochem. Cell Biol. 2016, 70, 92–104. [Google Scholar] [CrossRef] [PubMed]



| Sample | Cell Viability (Fold of Base) | Inhibition Percentage (%) |
|---|---|---|
| Control | 0.53 ± 0.01 | – |
| TGF | 1.65 ± 0.03 | – |
| TGF + PLE a | 1.19 ± 0.02 | 28% |
| TGF + 1 b | 0.54 ± 0.01 | 67% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-C.; Kuo, P.-C.; Hung, H.-Y.; Pan, T.-L.; Chen, F.-A.; Wu, T.-S. Ionone Derivatives from the Mycelium of Phellinus linteus and the Inhibitory Effect on Activated Rat Hepatic Stellate Cells. Int. J. Mol. Sci. 2016, 17, 681. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050681
Huang S-C, Kuo P-C, Hung H-Y, Pan T-L, Chen F-A, Wu T-S. Ionone Derivatives from the Mycelium of Phellinus linteus and the Inhibitory Effect on Activated Rat Hepatic Stellate Cells. International Journal of Molecular Sciences. 2016; 17(5):681. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050681
Chicago/Turabian StyleHuang, Shiow-Chyn, Ping-Chung Kuo, Hsin-Yi Hung, Tai-Long Pan, Fu-An Chen, and Tian-Shung Wu. 2016. "Ionone Derivatives from the Mycelium of Phellinus linteus and the Inhibitory Effect on Activated Rat Hepatic Stellate Cells" International Journal of Molecular Sciences 17, no. 5: 681. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050681