Antioxidant and Hepatoprotective Effects of Procyanidins from Wild Grape (Vitis amurensis) Seeds in Ethanol-Induced Cells and Rats
Abstract
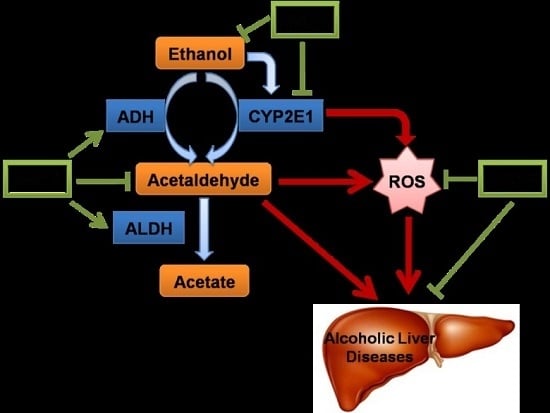
:1. Introduction
2. Results
2.1. Effect of Ethanol and Wild Grape Seed Procyanidins (WGP) on Viability of HepG2 Cells
2.2. Effect of WGP on the Cytochrome P450 2E1 (CYP2E1) Protein Level and Reactive Oxygen Species (ROS) Production in Ethanol-Treated HepG2 Cells
2.3. Effect of WGP on the Activity and Protein Expression of Antioxidant Enzymes in Ethanol-Treated HepG2 Cells
2.4. Effect of WGP on Phosphorylations of Upstream Kinases in Ethanol-Treated HepG2 Cells
2.5. Protective Effect of WGP Against Hepatotoxicity in Ethanol-Treated Rats
2.6. Effect of WGP on the Ethanol Metabolism Pathway in Rats
2.7. Effect of WGP on the Activity and Protein Expression of Antioxidant Enzymes in Ethanol-Treated Rats
2.8. Effect of WGP on Phosphorylation of Upstream Kinases in Ethanol-Treated Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of WGP
4.3. Cell Culture
4.4. Animal Experimental Design
4.5. Cell Viability Assay
4.6. ROS Formation Assay
4.7. Assessment of Antioxidant Enzymes Activity in HepG2 Cells and Liver Tissues
4.8. Assays of ADH and ALDH Activities
4.9. Measurements of Alcohol and Acetaldehyde Concentrations
4.10. AST and ALT Activities
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| ALDH | aldehyde dehydrogenase |
| ALT | alkaline phosphatase |
| AST | aspartate aminotransferase |
| CAT | catalase |
| CYP2E1 | cytochrome P450 2E1 |
| DCF-DA | 2′,7′-dichlorodihydrofluorescein |
| ERK | extracellular regulated kinase |
| GPx | glutathione peroxidase |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| SIL | silymarin |
| SOD | superoxide dismutase |
| WGP | wild grape seeds procyanidins |
References
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [PubMed]
- Surrenti, C.; Galli, M. Molecular mechanisms of alcohol-induced liver injury. An update. Minerva Gastroenterol. Dietol. 2003, 49, 95–105. [Google Scholar] [PubMed]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Swift, R. Direct measurement of alcohol and its metabolites. Addiction 2003, 98, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deaciuc, I.; Song, M.; Lee, D.Y.; Liu, Y.; Ji, X.; McClain, C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol. Clin. Exp. Res. 2006, 30, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [PubMed]
- Dey, A. Cytochrome P450 2E1: Its clinical aspects and a brief perpective on the current research scenario. Subcell. Biochem. 2013, 67, 1–104. [Google Scholar] [PubMed]
- Teschke, R.; Gellert, J. Hepatic microsomal ethanol-oxidizing system (MEOS): Metabolic aspects and clinical implications. Alcohol. Clin. Exp. Res. 1986, 10, 20S–32S. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Hasumura, Y.; Joly, J.G.; Lieber, C.S. Microsomal ethanol-oxidizing system (MEOS): Purification and properties of a rat liver system free of catalase and alcohol dehydrogenase. Biochem. Biophys. Res. Commun. 1972, 49, 1187–1193. [Google Scholar] [CrossRef]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. 2005, 569, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.; Viaggi, C.; Escrig, M.A.; Pascual, M.; Guerri, C.; Vaglini, F.; Aragon, C.M.; Corsini, G.U. Ethanol intake and ethanol-induced locomotion and locomotor sensitization in CYP2E1 knockout mice. Pharmacogenet. Genom. 2009, 19, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Protective effects of silymarin, a milk thistle (Silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J. Biochem. Biophys. 2006, 43, 306–311. [Google Scholar] [PubMed]
- Kim, Y.D.; Eom, S.Y.; Ogawa, M.; Oyama, T.; Isse, T.; Kang, J.W.; Zhang, Y.W.; Kawamoto, T.; Kim, H. Ethanol-induced oxidative DNA damage and CYP2E1 expression in liver tissue of ALDH2 knockout mice. J. Occup. Health 2007, 49, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, X.; Zhou, R.; Cederbaum, A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem. Biophys. Res. Commun. 2010, 402, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Dezfoulian, O.; Neamati, S.; Rashidipour, M.; Tanideh, N.; Kheradmand, A. Oleuropein prevents ethanol-induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J. Physiol. Biochem. 2012, 68, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Farbiszewski, R.; Radecka, A.; Chwiecko, M.; Holownia, A. The effect of heparegen on antioxidant enzyme activities in ethanol-induced liver injury in rats. Alcohol 1992, 9, 403–407. [Google Scholar] [CrossRef]
- Cho, M.H.; Shim, S.M.; Lee, S.R.; Mar, W.; Kim, G.H. Effect of Evodiae fructus extracts on gene expressions related with alcohol metabolism and antioxidation in ethanol-loaded mice. Food. Chem. Toxicol. 2005, 43, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- McDonough, K.H. Antioxidant nutrients and alcohol. Toxicology 2003, 189, 89–97. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.; Cederbaum, A. CYP2E1, oxidative stress and mapk signaling pathways in alcohol-induced hepatotoxicity. J. Biochem. Pharmacol. Res. 2014, 2, 74–90. [Google Scholar]
- Li, Y.; Gao, C.; Shi, Y.; Tang, Y.; Liu, L.; Xiong, T.; Du, M.; Xing, M.; Yao, P. Carbon monoxide alleviates ethanol-induced oxidative damage and inflammatory stress through activating p38 MAPK pathway. Toxicol. Appl. Pharmacol. 2013, 273, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Kim, S.J.; Mun, A.R.; Go, H.K.; Kim, G.B.; Kim, S.Z.; Jang, S.I.; Lee, S.J.; Kim, J.S.; Kang, H.S. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J. Ethnopharmacol. 2012, 141, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.R.; Kim, Y.H.; Gang, G.T.; Hwang, J.H.; Lee, H.S.; Ly, S.Y.; Oh, W.K.; Song, K.S.; Lee, C.H. Hepatoprotective effects of chestnut (Castanea crenata) inner shell extract against chronic ethanol-induced oxidative stress in C57BL/6 mice. Food Chem. Toxicol. 2011, 49, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszen, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, C.; Ren, G. Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J. Agric. Food Chem. 2011, 59, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Jun, M.; Jeong, W.S. Procyanidins from wild grape (Vitis amurensis) seeds regulate are-mediated enzyme expression via Nrf2 coupled with p38 and PI3K/Akt pathway in HepG2 cells. Int. J. Mol. Sci. 2012, 13, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Truong, V.L.; Kang, H.S.; Jun, M.; Jeong, W.S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid. Med. Cell. Longev. 2013, 2013, 409321–409331. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Das, S.K.; Vasudevan, D.M. Dietary grapes (Vitis vinifera) feeding attenuates ethanol-induced oxidative stress in blood and modulates immune functions in mice. Indian J. Biochem. Biophys. 2012, 49, 379–385. [Google Scholar] [PubMed]
- Jimenez-Lopez, J.M.; Cederbaum, A.I. Green tea polyphenol epigallocatechin-3-gallate protects hepg2 cells against CYP2E1-dependent toxicity. Free Radic. Biol. Med. 2004, 36, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; El May, M.; Gharbi, N.; Kamoun, A.; El-Fazaa, S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007, 80, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Bak, M.J.; Jun, M.; Kong, A.N.; Ho, C.T.; Jeong, W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, L.H.; Sun, B.; Yang, J.Y.; Zhao, Y.Q.; Dong, Y.X.; Spranger, M.I.; Wu, C.F. Direct in vivo evidence of protective effects of grape seed procyanidin fractions and other antioxidants against ethanol-induced oxidative DNA damage in mouse brain cells. J. Agric. Food Chem. 2007, 55, 5881–5891. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Akiyama, H.; Kanda, T.; Ohtake, Y.; Goda, Y. Apple procyanidin oligomers absorption in rats after oral administration: Analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [PubMed]
- Zhang, C.; Tian, X.; Luo, Y.; Meng, X. Ginkgolide B attenuates ethanol-induced neurotoxicity through regulating nadph oxidases. Toxicology 2011, 287, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kang, H.S.; Yun, C.H.; Kwak, H.S. Potential in vitro protective effect of quercetin, catechin, caffeic acid and phytic acid against ethanol-induced oxidative stress in SK-Hep-1 cells. Biomol. Ther. 2012, 20, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.A.; Harikrishnan, R.; Indira, M. Ascorbic acid is superior to silymarin in the recovery of ethanol-induced inflammatory reactions in hepatocytes of guinea pigs. J. Physiol. Biochem. 2013, 69, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Monzoni, A.; Masutti, F.; Saccoccio, G.; Bellentani, S.; Tiribelli, C.; Giacca, M. Genetic determinants of ethanol-induced liver damage. Mol. Med. 2001, 7, 255–262. [Google Scholar] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.; Ashar, H.; Srinath, S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol-induced hepatic damage in rodents. Interdiscip. Toxicol. 2012, 5, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Jun, M.; Jeong, W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- Drever, N.; Yin, H.; Kechichian, T.; Costantine, M.; Longo, M.; Saade, G.R.; Bytautiene, E. The expression of antioxidant enzymes in a mouse model of fetal alcohol syndrome. Am. J. Obstet. Gynecol. 2012, 206, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Ande, A.; Kumar, A.; Kumar, S. Regulation of cytochrome P450 2E1 expression by ethanol: Role of oxidative stress-mediated PKC/JNK/SP1 pathway. Cell Death Dis. 2013, 4, e554. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Bardag-Gorce, F.; Tillman, B.; French, S.W. Protective effect of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Exp. Mol. Pathol. 2011, 90, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, W.; Yao, P.; Zhang, B.; Sun, S.; Nussler, A.K.; Liu, L. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol. in Vitro 2010, 24, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.L.; So, K.F.; Tipoe, G.L. Zeaxanthin dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating mapk pathway. PLoS ONE 2014, 9, e95214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. PI3k/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology 2012, 296, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Carrillo, M.C.; Kanai, S.; Nokubo, M.; Kitani, K. (−) deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 1991, 48, 517–521. [Google Scholar] [CrossRef]
- Bogdanska, J.J.; Korneti, P.; Todorova, B. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in republic of macedonia. Bratisl. Lek. Listy 2003, 104, 108–114. [Google Scholar] [PubMed]








| Groups | Initial Body Weight (g) | Final Body Weight (g) | Liver Weight (g) | Relative Liver Weight (Liver/100 g Body Weight) |
|---|---|---|---|---|
| Control | 188.94 ± 5.61 | 311.34 ± 9.17 | 12.7 ± 0.55 | 4.08 ± 0.11 |
| WGP (50 mg/kg) | 171.80 ± 6.12 | 315.67 ± 9.95 | 13.01 ± 2.16 | 4.17 ± 0.90 |
| Ethanol (EtOH) (6 g/kg) | 179.18 ± 3.69 | 312.30 ± 13.69 | 16.10 ± 2.06 * | 5.18 ± 0.86 * |
| WGP (10 mg/kg) + EtOH | 180.84 ± 6.26 | 313.65 ± 12.65 | 13.20 ± 1.84 # | 4.20 ± 0.51 # |
| WGP (50 mg/kg) + EtOH | 178.28 ± 6.80 | 320.62 ± 14.22 | 13.20 ± 0.83 # | 4.12 ± 0.16 # |
| Silymarin (SIL) (50 mg/kg) + EtOH | 179.92 ± 5.42 | 323.75 ± 8.48 | 12.78 ± 1.71 # | 3.94 ± 0.46 # |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, M.J.; Truong, V.-L.; Ko, S.-Y.; Nguyen, X.N.G.; Ingkasupart, P.; Jun, M.; Shin, J.Y.; Jeong, W.-S. Antioxidant and Hepatoprotective Effects of Procyanidins from Wild Grape (Vitis amurensis) Seeds in Ethanol-Induced Cells and Rats. Int. J. Mol. Sci. 2016, 17, 758. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050758
Bak MJ, Truong V-L, Ko S-Y, Nguyen XNG, Ingkasupart P, Jun M, Shin JY, Jeong W-S. Antioxidant and Hepatoprotective Effects of Procyanidins from Wild Grape (Vitis amurensis) Seeds in Ethanol-Induced Cells and Rats. International Journal of Molecular Sciences. 2016; 17(5):758. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050758
Chicago/Turabian StyleBak, Min Ji, Van-Long Truong, Se-Yeon Ko, Xuan Ngan Giang Nguyen, Pajaree Ingkasupart, Mira Jun, Jin Young Shin, and Woo-Sik Jeong. 2016. "Antioxidant and Hepatoprotective Effects of Procyanidins from Wild Grape (Vitis amurensis) Seeds in Ethanol-Induced Cells and Rats" International Journal of Molecular Sciences 17, no. 5: 758. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050758