3.4.2. Ultrasound Method
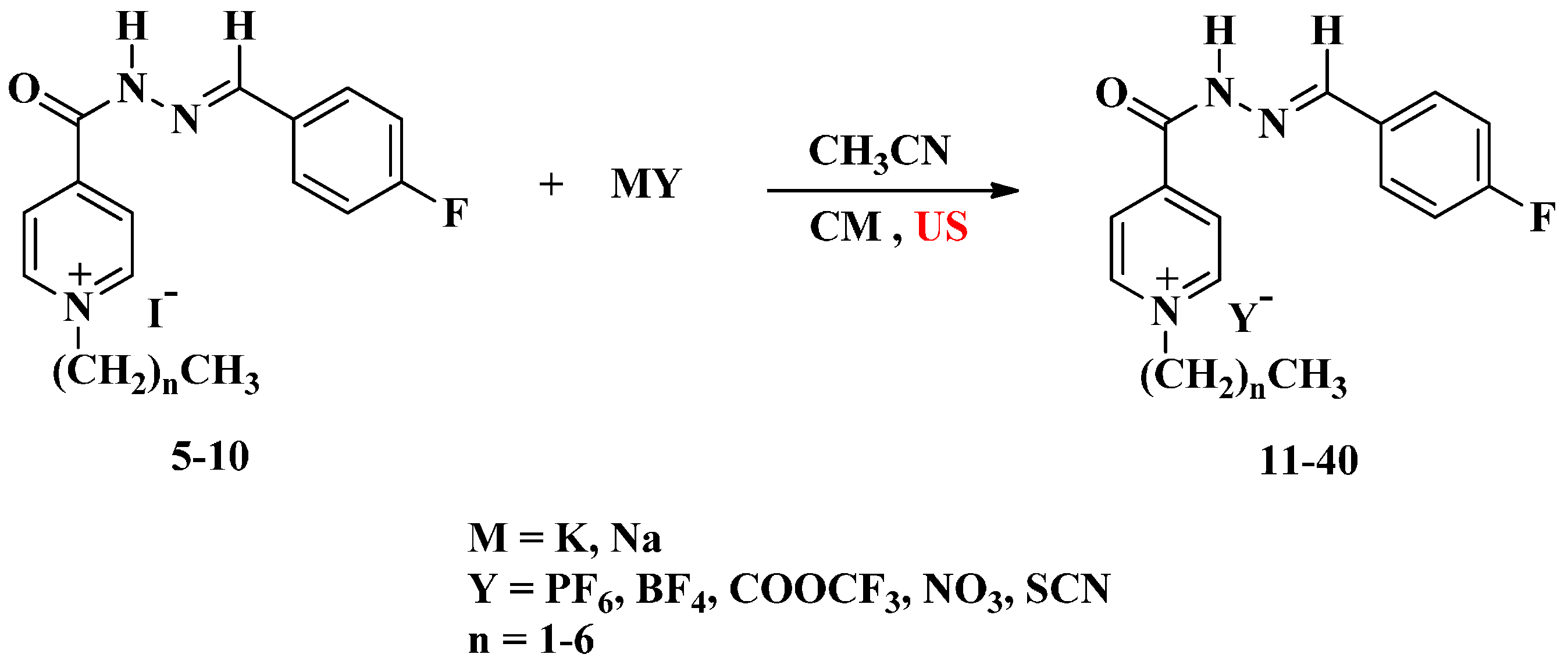
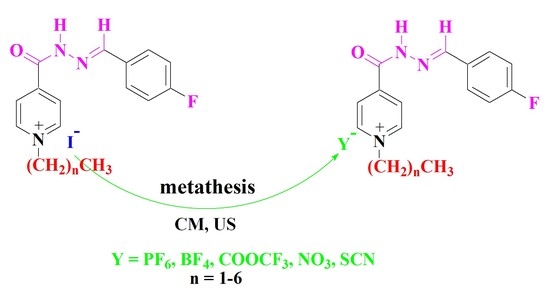
A mixture of compounds 5–10 (1 mmol) in acetonitrile (8 mL) and potassium hexafluorophosphate, sodium tetrafluoroborate, sodium nitrate, sodium thiocyanate and/or sodium trifluoroacetate (1.2 mmol) was irradiated by ultrasound irradiation for 5–6 h at room temperature. The reaction proceeded as described above to afford the same compounds 11–40.
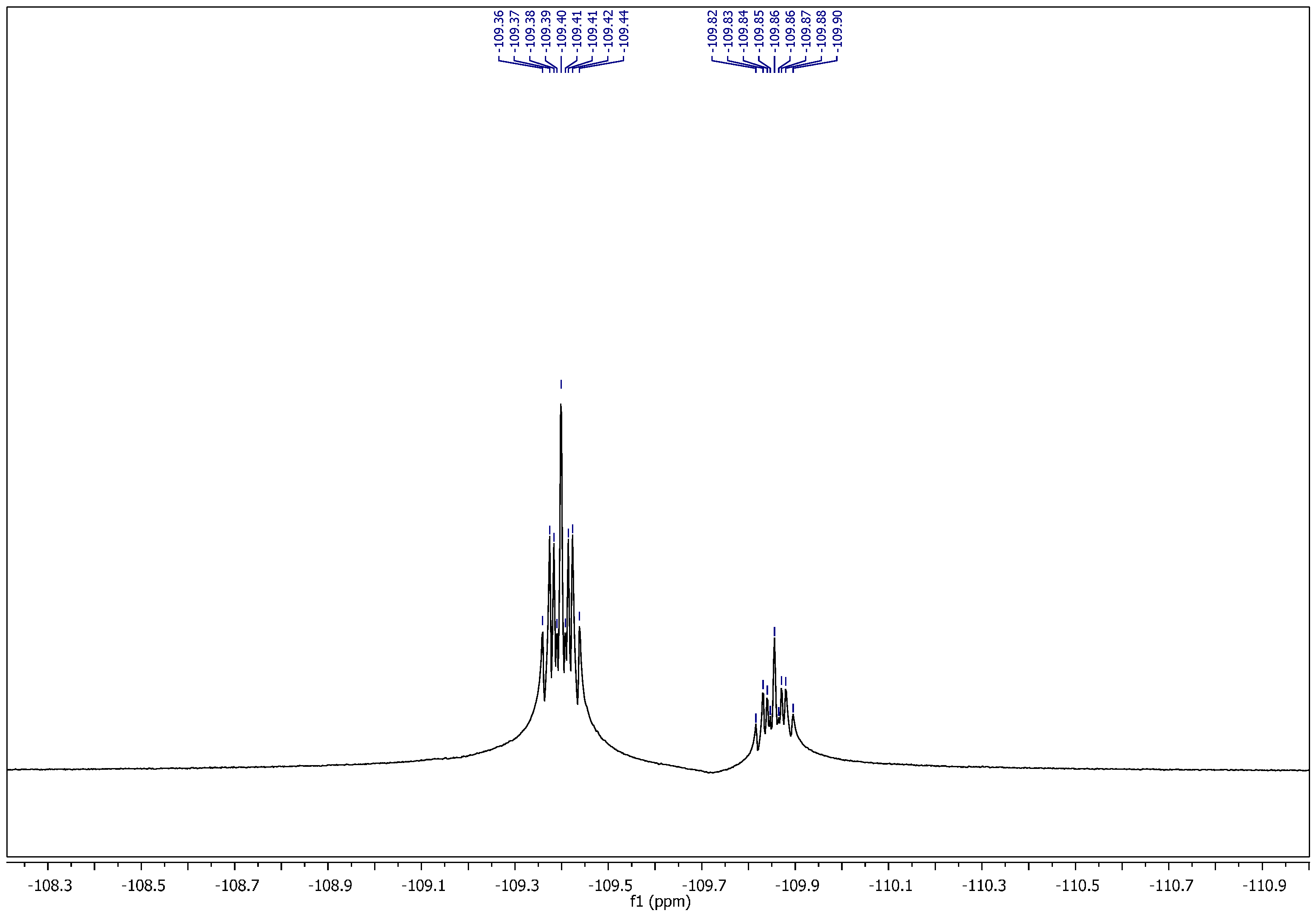
1-Ethyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium hexafluorophosphate (11). It was obtained as yellow crystals; mp: 175–176 °C. 1H NMR (400 MHz, DMSO-d6): δH = 1.57–1.63 (m, 3H, CH3), 4.69–4.74 (q, 2H, J = 4 Hz, 8 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 16.00, 16.12 (CH3), 56.51, 56.61 (NCH2), 115.71, 115.89, 116.11, 126.05, 127.08, 129.32, 129.41, 129.66, 129.74, 129.98, 130.16, 130.19, 144.79, 145.09, 145.43, 147.27, 149.32, 149.46 (Ar–C), 158.72, 161.91, 162.22, 164.69, 165.13 (C=N, C=O). 31P NMR (162 MHz, DMSO-d6): δP = −157.37 to −131.03 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6): δF = −71.09, −69.20 (2s, 6F, PF6), (−109.89 to −109.81), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 417.95 [M+].
1-Ethyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium tetrafluoroborate (12). It was obtained as yellow crystals; mp: 205–206 °C. 1H NMR (400 MHz, DMSO-d6): δH = 1.57–1.63 (m, 3H, CH3), 4.69–4.74 (q, 2H, J = 4 Hz, 8 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.48 (s, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 16.06, 16.19 (CH3), 56.57, 56.67 (NCH2), 115.77, 115.96, 116.18, 126.11, 127.13, 129.39, 129.47, 129.72, 129.81, 130.04, 130.21, 130.24, 144.86, 145.14, 145.50, 147.30, 149.37, 149.51 (Ar–C), 158.77, 162.28, 164.76, 165.20 (C=N, C=O). 11B NMR (128 MHz, DMSO-d6): δB = −1.29 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.85 to −109.82), (−109.42 to −109.34) (2m, 1F, Ar–F), −148.28, −148.29 (2d, 4F, BF4). MS (ES) m/z = 359.46 [M+].
1-Ethyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium trifluoroacetate (13). It was obtained as yellow crystals; mp: 211–212 °C. 1H NMR (400 MHz, DMSO-d6): δH = 1.57–1.63 (m, 3H, CH3), 4.69–4.75 (q, 2H, J = 8 Hz, 12 Hz, NCH2), 7.27 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 4 Hz, Ar–H), 9.25 (d, 0.5H, J = 4 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 16.00, 16.13 (CH3), 56.50, 56.59 (NCH2), 115.71, 115.89, 116.11, 126.03, 127.06, 129.33, 129.41, 129.64, 129.73, 129.97, 130.16, 130.19, 144.80, 145.07, 145.42, 147.29, 149.30, 149.44 (Ar–C), 158.73, 161.89, 162.20, 164.67, 165.12 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.49 (s, 3F, CF3), (−109.89 to −109.82), (−109.45 to −109.37) (2m, 1F, Ar–F). MS (ES) m/z = 385.22 [M+].
1-Ethyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium nitrate (14). It was obtained as yellow crystals; mp: 210–211 °C. 1H NMR (400 MHz, DMSO-d6): δH = 1.57–1.63 (m, 3H, CH3), 4.69–4.74 (q, 2H, J = 8 Hz, 12 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 4 Hz, Ar–H), 9.25 (d, 0.5H, J = 4 Hz, Ar–H), 9.34 (d, 1.5H, J = 4 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 16.00, 16.13 (CH3), 56.50, 56.59 (NCH2), 115.71, 115.89, 116.11, 126.03, 127.06, 129.33, 129.41, 126.65, 129.73, 129.97, 130.16, 130.19, 144.81, 145.08, 145.46, 147.24, 149.30, 149.44 (Ar–C), 158.72, 161.89, 162.21, 164.36, 164.68, 165.13 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 334.37 [M+].
1-Ethyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium thiocyanate (15). It was obtained as yellow crystals; mp: 203–205 °C. 1H NMR (400 MHz, DMSO-d6): δH = 1.58–1.63 (m, 3H, CH3), 4.69–4.75 (q, 2H, J = 8 Hz, 12 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 16.01, 16.12 (CH3), 56.52, 56.62 (NCH2), 115.70, 115.89, 116.11, 126.04, 127.07, 129.32, 129.40, 129.50, 129.65, 129.74, 129.95, 130.15, 130.18, 144.79, 145.09, 145.44, 147.24, 149.31, 149.45 (Ar–C), 158.72, 162.21, 164.69, 165.12 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6) δF = (−109.89 to −109.81), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 330.17 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridinium hexafluorophosphate (16). It was obtained as yellow crystals; mp: 180–181 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.89–0.95 (m, 3H, CH3), 1.94–2.05 (m, 2H, NCH2CH2), 4.67 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.47 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 11.16, 11.19 (CH3), 25.00, 25.08 (NCH2CH2), 63.22, 63.30 (NCH2), 116.75, 116.94, 117.16, 127.13, 128.09, 130.33, 130.41, 130.71, 130.79, 130.99, 131.18, 131.21, 146.06, 146.67, 148.34, 150.32, 150.64 (Ar–C), 159.75, 163.26, 165.73, 166.19 (C=N, C=O).31P NMR (162 MHz, DMSO-d6): δP = −152.98 to −135.42 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6): δF = −71.08, −69.19, (2s, 6F, PF6), (−109.89 to −109.83), (−109.41 to −109.33) (2m, 1F, Ar–F). MS (ES) m/z = 431.37 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridinium tetrafluoroborate (17). It was obtained as yellow crystals; mp: 162–163 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.90–0.95 (m, 3H, CH3), 1.94–2.05 (m, 2H, NCH2CH2), 4.65 (t, 2H, J = 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.23 (d, 0.5H, J = 8 Hz, Ar–H), 9.31 (d, 1.5H, J = 4 Hz, Ar–H), 12.46 (s, 0.75H, CONH), 12.50 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 10.18 (CH3), 24.02, 24.10 (NCH2CH2), 62.26, 62.35 (NCH2), 115.77, 115.96, 116.18, 126.15, 127.12, 129.34, 129.43, 129.73, 129.82, 130.05, 130.20, 130.23, 145.10, 145.69, 147.39, 149.36 (Ar–C), 158.78, 162.29, 164.76, 165.21 (C=N, C=O). 11B NMR (128 MHz, DMSO-d6): δB = −1.29 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.80), (−109.41 to −109.33) (2m, 1F, Ar–F); −148.29, −148.24 (2d, 4F, BF4). MS (ESI) m/z = 373.49 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridinium trifluoroacetate (18). It was obtained as yellow crystals; mp: 151–152 °C. 1H NMR (400 MHz, DMSO-d6): δH =0.89–0.95 (m, 3H, CH3), 1.94–2.03 (m, 2H, NCH2CH2), 4.65 (t, 2H, J = 8 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 10.12, 10.15 (CH3), 23.97, 24.06 (NCH2CH2), 62.15, 62.24 (NCH2), 115.71, 115.90, 116.12, 126.08, 127.04, 129.27, 129.36, 129.66, 129.74, 129.97, 130.15, 144.99, 145.62, 147.29, 149.23 (Ar–C), 158.71, 162.21, 165.17 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.47 (s, 3F, CF3), (−109.87 to −109.79), (−109.40 to −109.31) (2m, 1F, Ar–F). MS (ES) m/z = 399.00 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridinium nitrate (19). It was obtained as yellow crystals; mp: 172–173 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.90–0.95 (m, 3H, CH3), 1.94–2.05 (m, 2H, NCH2CH2), 4.69 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.25 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.36 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.52 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.26 (d, 0.5H, J = 8 Hz, Ar–H), 9.35 (d, 1.5H, J = 4 Hz, Ar–H), 12.45 (ds, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 8.49 (CH3), 22.31, 22.41 (NCH2CH2), 60.58, 60.64 (NCH2), 114.08, 114.26, 114.48, 124.45, 125.43, 127.69, 127.78, 128.04, 128.13, 128.35, 128.51, 128.54, 143.38, 143.43, 14.00, 145.65, 147.72, 147.96 (Ar–C), 157.06, 160.26, 160.59, 162.73, 163.06, 163.46 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.80), (−109.41 to −109.33) (2m, 1F, Ar–F). MS (ES) m/z = 348.29 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridinium thiocyanate (20). It was obtained as yellow crystals; mp: 160–161 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.91–0.96 (m, 3H, CH3), 1.96–2.05 (m, 2H, NCH2CH2), 4.68 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.42 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.55 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.32 (d, 1.5H, J = 4 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 10.20 (CH3), 25.04, 24.12 (NCH2CH2), 62.28, 62.36 (NCH2), 115.76, 115.95, 116.16, 126.15, 127.13, 129.35, 129.44, 129.65, 129.72, 129.81, 130.02, 130.05, 130.22, 130.25, 145.10, 145.67, 147.40, 149.36, 149.66 (Ar–C), 158.79, 161.96, 162.27, 164.42, 164.74, 165.19 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.83), (−109.41 to −109.33) (2m, 1F, Ar–F). MS (ES) m/z = 344.48 [M+].
1-Butyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium hexafluorophosphate (21). It was obtained as yellow crystals; mp: 145–146 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.91–0.96 (m, 3H, CH3), 1.28–1.39 (m, 2H, CH2CH3), 1.91–2.00 (m, 2H, NCH2CH2), 4.70 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.26 (t, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.46 (s, 0.75H, CONH), 12.51 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.27, 13.30 (CH3), 18.73, 18.80 (CH2CH3), 32.48, 32.57 (NCH2CH2), 60.79 (NCH2), 115.77, 115.96, 116.18, 126.16, 127.13, 129.35, 129.44, 129.73, 129.82, 130.05, 130.20, 130.23, 145.08, 145.69, 147.33, 149.34 (Ar–C), 158.76, 162.28, 164.76, 165.21 (C=N, C=O). 31P NMR (162 MHz, DMSO-d6): δP = −152.98 to −135.42 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6): δF = −71.09, −69.19 (2s, 6F, PF6), (−109.88 to −109.84), (−109.41 to −109.33) (2m, 1F, Ar–F). MS (ES) m/z = 445.02 [M+].
1-Butyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridiniumtetrafluoroborate (22). It was obtained as yellow crystals; mp: 172–173 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.92–0.97 (m, 3H, CH3), 1.28–1.39 (m, 2H, CH2CH3), 1.92–2.01 (m, 2H, NCH2CH2), 4.71 (t, 2H, J = 8 Hz, NCH2), 7.27 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.90 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 4 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.55 (d, 1.5H, J = 8 Hz, Ar–H), 9.27 (d, 0.5H, J = 4 Hz, Ar–H), 9.36 (d, 1.5H, J = 4 Hz, Ar–H), 12.49 (s, 0.75H, CONH), 12.53 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 11.76 (CH3), 17.20, 17.27 (CH2CH3), 30.96, 31.06 (NCH2CH2), 59.19, 59.24 (NCH2), 114.25, 114.44, 114.66, 124.62, 125.59, 127.84, 127.93, 128.20, 128.29, 128.50, 128.66, 128.69, 143.54, 144.17, 145.75, 147.79, 148.07 (Ar–C), 157.23, 160.41, 160.74, 163.21, 164.68 (C=N, C=O). 11B NMR (128 MHz, DMSO-d6): δB = −1.29 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.40 to −109.33) (2m, 1F, Ar–F); −148.22, −148.16 (2d, 4F, BF4). MS (ES) m/z = 387.27 [M+].
1-Butyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium trifluoroacetate (23). It was obtained as yellow crystals; mp: 178–179 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.91–0.96 (m, 3H, CH3), 1.28–1.37 (m, 2H, CH2CH3), 1.92–2.01 (m, 2H, NCH2CH2), 4.71 (t, 2H, J = 8 Hz, NCH2), 7.25 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.36 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.88 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.27 (d, 0.5H, J = 8 Hz, Ar–H), 9.36 (d, 1.5H, J = 8 Hz, Ar–H), 12.45 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.26, 13.28 (CH3), 18.71, 18.78 (CH2CH3), 32.45, 32.55 (NCH2CH2), 60.75, 60.79 (NCH2), 115.76, 115.94, 116.16, 126.14, 127.12, 129.38, 129.47, 129.72, 129.81, 130.01, 130.21, 130.24, 145.11, 145.70, 147.31, 149.40, 149.62 (Ar–C), 158.93, 161.95, 162.27, 164.42, 164.74, 165.14 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.47 (s, 3F, CF3), (−109.87 to −109.81), (−109.40 to −109.32) (2m, 1F, Ar–F). MS (ESI) m/z = 414.00 [M+ + H].
1-Butyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium nitrate (24). It was obtained as yellow crystals; mp: 170–171 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.92–0.97 (m, 3H, CH3), 1.29–1.38 (m, 2H, CH2CH3), 1.92–2.02 (m, 2H, NCH2CH2), 4.71 (t, 2H, J = 8 Hz, NCH2), 7.27 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.90 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.17 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 4 Hz, Ar–H), 8.52 (s, 0.75H, H–C=N), 8.55 (d, 1.5H, J = 8 Hz, Ar–H), 9.28 (d, 0.5H, J = 8 Hz, Ar–H), 9.36 (d, 1.5H, J = 4 Hz, Ar–H), 12.49 (s, 0.75H, CONH), 12.53 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 15.03, 15.06 (CH3), 20.47, 20.53 (CH2CH3), 34.22, 34.32 (NCH2CH2), 62.46, 62.51 (NCH2), 117.52, 117.71, 117.93, 127.89, 128.86, 131.12, 131.20, 131.47, 131.56, 131.77, 131.93, 131.96, 146.81, 147.44, 149.02, 151.06, 151.34 (Ar–C), 160.50, 163.68, 164.01, 166.15, 166.48, 166.94 (C=N, C=O).19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.40 to −109.33) (2m, 1F, Ar–F). MS (ESI) m/z = 362.27 [M+].
1-Butyl-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)pyridinium thiocyanate (25). It was obtained as yellow crystals; mp: 172–173 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.92−0.96 (m, 3H, CH3), 1.28−1.37 (m, 2H, CH2CH3), 1.92−2.01 (m, 2H, NCH2CH2), 4.72 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.24 (t, 0.5H, J = 8 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 4 Hz, Ar–H), 9.27 (d, 0.5H, J = 8 Hz, Ar–H), 9.36 (d, 1.5H, J = 8 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.46, 13.48 (CH3), 18.89, 18.96 (CH2CH3), 32.65, 32.74 (NCH2CH2), 60.88, 60.94 (NCH2), 115.94, 116.13, 116.35, 126.32, 127.29, 129.53, 129.62, 129.90, 129.99, 130.20, 130.36, 130.39, 145.24, 145.87, 147.46, 149.49, 149.77 (Ar–C), 158.93, 162.44, 164.91, 165.38 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.88 to −109.84), (−109.41 to −109.33) (2m, 1F, Ar–F). MS (ES) m/z = 358.26 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-pentylpyridinium hexafluorophosphate (26). It was obtained as yellow crystals; mp: 195–196 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86−0.91 (m, 3H, CH3), 1.23−1.37 (m, 4H, 2×CH2), 1.93–2.02 (m, 2H, NCH2CH2), 4.69 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.24 (d, 0.5H, J = 4 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.46 (s, 0.75H, CONH), 12.51 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.69 (CH3), 21.50, 27.47, 27.55 (2×CH2), 30.20, 30.31 (NCH2CH2), 60.92, 61.00 (NCH2), 115.76, 115.96, 116.18, 126.16, 127.12, 129.34, 129.43, 129.73, 129.81, 130.20, 130.23, 145.09, 145.69, 147.34, 149.34 (Ar–C), 158.76, 162.28, 164.76, 165.22 (C=N, C=O).31P NMR (162 MHz, DMSO-d6): δP = −152.98 to −135.42 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6): δF = −69.21, −71.10 (2s, 6F, PF6), (−109.89 to −109.81), (−109.41 to −109.34) (2m, 1F, Ar–F). MS (ES) m/z = 459.90 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-pentylpyridinium tetrafluoroborate (27). It was obtained as yellow crystals; mp: 205–206 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.90 (m, 3H, CH3), 1.23–1.37 (m, 4H, 2×CH2), 1.93–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 4 Hz, 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 4 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.47 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70 (CH3), 21.51, 27.47, 27.55 (2×CH2), 30.20, 30.31 (NCH2CH2), 60.99 (NCH2), 115.76, 115.96, 116.18, 126.15, 127.11, 129.35, 129.44, 129.73, 129.82, 130.20, 130.23, 145.08, 145.68, 147.32, 149.33, 149.63(Ar–C), 158.76, 162.28, 164.75, 165.22 (C=N, C=O).11B NMR (128 MHz, DMSO-d6): δB = −1.29 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.42 to −109.34) (2m, 1F, Ar–F); −148.28, −148.23 (2d, 4F, BF4). MS (ES) m/z = 401.00 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-pentylpyridinium trifluoroacetate (28). It was obtained as yellow crystals; mp: 201–202 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.90 (m, 3H, CH3), 1.23–1.37 (m, 4H, 2×CH2), 1.93–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.48 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70, 13.72 (CH3), 21.51, 27.47, 27.55 (2×CH2), 30.20, 30.31 (NCH2CH2), 60.92, 60.99 (NCH2), 115.76, 115.96, 116.18, 126.15, 127.11, 129.35, 129.44, 129.73, 129.81, 130.21, 130.24, 145.08, 145.69, 147.34, 149.33 (Ar–C), 158.77, 162.28, 164.75, 165.22 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.50 (s, 3F, CF3), (−109.87 to −109.83), (−109.42 to −109.35) (2m, 1F, Ar–F). MS (ESI) m/z = 428.00 [M+ + H].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-pentylpyridinium nitrate (29). It was obtained as yellow crystals; mp: 200–201 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.90 (m, 3H, CH3), 1.23–1.37 (m, 4H, 2×CH2), 1.93–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.47 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70, 1371 (CH3), 21.51, 24.47, 27.55 (2×CH2), 30.20, 30.31 (NCH2CH2), 60.91, 60.99 (NCH2), 115.76, 115.97, 116.19, 126.15, 127.12, 129.35, 129.44, 129.73, 129.82, 130.20, 130.23, 145.08, 145.69, 147.33, 149.33 (Ar–C), 158.76, 162.28, 164.75, 165.22 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.42 to −109.34) (2m, 1F, Ar–F). MS (ES) m/z = 376.70 [M+].
4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)-1-pentylpyridinium thiocyanate (30). It was obtained as yellow crystals; mp: 197–198 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.90 (m, 3H, CH3), 1.23–1.37 (m, 4H, 2×CH2), 1.93–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.15 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.47 (s, 0.75H, CONH), 12.51 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70, 1371 (CH3), 21.51, 27.47, 27.55 (2×CH2), 30.20, 30.31 (NCH2CH2), 60.92, 61.00 (NCH2), 115.76, 115.96, 116.18, 126.15, 127.12, 129.36, 129.44, 129.73, 129.82, 130..02, 130.20, 130.23, 145.08, 145.69, 147.32, 149.34, 149.63(Ar–C), 158.76, 162.28, 164.75, 165.21 (C=N, C=O).19F NMR (377 MHz, DMSO-d6): δF = (−109.89 to −109.81), (−109.42 to −109.34) (2m, 1F, Ar–F). MS (ES) m/z = 372.00 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-hexylpyridinium hexafluorophosphate (31). It was obtained as yellow crystals; mp: 143–144 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.93 (m, 3H, CH3), 1.28–1.34 (m, 6H, 3×CH2), 1.94–2.00 (m, 2H, NCH2CH2), 4.69 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.25 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.37 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 4 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 4 Hz, Ar–H), 9.24 (d, 0.5H, J = 8 Hz, Ar–H), 9.31 (d, 1.5H, J = 4 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.74, 13.76 (CH3), 21.80, 25.01, 25.04, 30.50, 30.58 (4×CH2), 60.96, 61.04 (NCH2), 115.73, 115.94, 116.16, 126.15, 127.14, 129.33, 129.42, 129.72, 129.80, 130.06, 130.20, 130.25, 145.04, 145.12, 145.65, 147.40, 149.37, 149.65 (Ar–C), 158.77, 161.96, 162.28, 164.43, 164.76, 165.20 (C=N, C=O). 31P NMR (162 MHz, DMSO-d6) δP = −157.37 to −131.02 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6): δF = −71.10, −69.22 (2s, 6F, PF6), (−109.90 to −109.82), (−109.44 to −109.36) (2m, 1F, Ar–F). MS (ES) m/z = 473.39 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-hexylpyridinium tetrafluoroborate (32). It was obtained as yellow crystals; mp: 204–205 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.92 (m, 3H, CH3), 1.29–1.30 (m, 6H, 3×CH2), 1.94–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 4 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.48 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70, 13.72 (CH3), 21.75, 24.95, 24.99, 30.40, 30.45, 30.53 (4×CH2), 60.89, 60.97 (NCH2), 115.69, 115.89, 116.11, 126.09, 127.07, 129.29, 129.38, 129.66, 129.75, 129.98, 130.17, 130.20, 145.01, 145.61, 147.34, 149.31, 149.59 (Ar–C), 158.72, 162.22, 164.70, 165.15 (C=N, C=O). 11B NMR (128 MHz, DMSO-d6): δB = −1.28 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.90 to −109.82), (−109.45 to −109.37) (2m, 1F, Ar–F); −148.30, −145.25 (2d, 4F, BF4). MS (ES) m/z = 415.22 [M+].
4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)-1-hexylpyridinium trifluoroacetate (33). It was obtained as yellow crystals; mp: 214–215 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.92 (m, 3H, CH3), 1.28–1.30 (m, 6H, 3×CH2), 1.94–2.00 (m, 2H, NCH2CH2), 4.68 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.76, 13.79 (CH3), 21.81, 25.01, 25.04, 30.50, 30.59 (4×CH2), 60.93, 61.00 (NCH2), 115.75, 115.96, 116.17, 126.14, 127.12, 129.35, 129.43, 129.71, 129.80, 130.03, 130.24, 130.27, 145.09, 145.66, 147.43, 149.34, 149.64 (Ar–C), 158.80, 162.27, 164.42, 164.74, 165.22 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.50 (s, 3F, CF3), (−109.90 to −109.82), (−109.46 to −109.38) (2m, 1F, Ar–F). MS (ES) m/z = 441.18 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-hexylpyridinium nitrate (34). It was obtained as yellow crystals; mp: 214–215 °C1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.92 (m, 3H, CH3), 1.28–1.33 (m, 6H, 3×CH2), 1.94–2.00 (m, 2H, NCH2CH2), 4.70 (t, 2H, J = 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.17 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 4 Hz, Ar–H), 9.27 (d, 0.5H, J = 8 Hz, Ar–H), 9.36 (d, 1.5H, J = 8 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.76, 13.78 (CH3), 21.81, 25.01, 25.04, 30.46, 30.50, 30.59 (4×CH2), 60.93, 60.99 (NCH2), 115.75, 115.95, 116.17, 126.14, 127.12, 129.36, 129.45, 129.72, 129.80, 130.05, 130.23, 130.26, 145.08, 145.68, 147.37, 149.35, 149.62 (Ar–C), 158.78, 161.94, 162.26, 164.41, 164.73, 165.20 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.90 to −109.82), (−109.45 to −109.37) (2m, 1F, Ar–F). MS (ESI) m/z = 390.37 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-hexylpyridinium thiocyanate (35). It was obtained as yellow crystals; mp: 189–190 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.86–0.92 (m, 3H, CH3), 1.28–1.33 (m, 6H, 3×CH2), 1.94–2.00 (m, 2H, NCH2CH2), 4.70 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.70, 13.72 (CH3), 21.75, 24.96, 24.99, 30.45, 30.54 (4×CH2), 60.89, 60.97 (NCH2), 115.69, 115.89, 116.11, 126.09, 127.07,129.29, 129.37, 129.52, 129.75, 130.00, 130.17, 130.20, 145.01, 145.61, 147.32, 149.29, 149.57 (Ar–C), 158.72,161.89, 162.21, 164.36, 164.69, 165.15 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.90 to −109.82), (−109.45 to −109.37) (2m, 1F, Ar–F). MS (ES) m/z = 386.56 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-heptylpyridinium hexafluorophosphate (36). It was obtained as yellow crystals; mp: 209–210 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.85–0.88 (m, 3H, CH3), 1.25–1.31 (m, 8H, 4×CH2), 1.91–2.00 (m, 2H, NCH2CH2), 4.69 (t, 2H, J = 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.35 (t, 1.5H, J = 8 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 4 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 8 Hz, Ar–H), 9.25 (d, 0.5H, J = 4 Hz, Ar–H), 9.34 (d, 1.5H, J = 8 Hz, Ar–H), 12.49 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 13.87 (CH3), 21.91, 25.31, 25.36, 28.00, 30.51, 30.64, 30.96, 30.98 (5×CH2), 60.94, 61.01 (NCH2), 115.75, 115.96, 116.18, 126.14, 127.11, 126.36, 129.44, 129.73, 129.81, 130.05, 130.21, 130.24, 145.07, 145.67, 147.33, 149.34, 149.63 (Ar–C), 158.77, 162.28, 164.75, 165.22 (C=N, C=O). 31P NMR (162 MHz, DMSO-d6): δP = −157.37 to −131.02 (m, 1P, PF6). 19F NMR (377 MHz, DMSO-d6) δF = −71.10, −69.21 (2s, 6F, PF6), (−109.93 to −109.85), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 487.37 [M+].
4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)-1-heptylpyridinium tetrafluoroborate (37). It was obtained as yellow crystals; mp: 203–204 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.85–0.89 (m, 3H, CH3), 1.25–1.31 (m, 8H, 4×CH2), 1.92–2.01 (m, 2H, NCH2CH2), 4.71 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.90 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 4 Hz, Ar–H), 9.27 (d, 0.5H, J = 8 Hz, Ar–H), 9.35 (d, 1.5H, J = 4 Hz, Ar–H), 12.49 (s, 0.75H, CONH), 12.53 (s, 0.25H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 14.04 (CH3), 22.09, 25.47, 25.53, 28.17, 30.68, 30.81, 31.12, 31.15 (5×CH2), 61.09, 61.16 (NCH2), 115.92, 116.13, 116.35, 126.31, 127.27, 129.53, 129.62, 129.90, 129.99, 130.18, 130.35, 130.38, 145.23, 145.84, 147.43, 149.49, 149.77 (Ar–C), 158.93, 162.09, 162.43, 164.56, 164.91, 165.39 (C=N, C=O). 11B NMR (128 MHz, DMSO-d6): δB = −1.14 (d, 1B, BF4). 19F NMR (377 MHz, DMSO-d6): δF = (−109.76 to −109.69), (−109.25 to −109.17) (2m, 1F, Ar–F); −148.08, −148.03 (2d, 4F, BF4). MS (ES) m/z = 429.21 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-heptylpyridinium trifluoroacetate (38). It was obtained as yellow crystals; mp: 203–204 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.85–0.88 (m, 3H, CH3), 1.23–1.31 (m, 8H, 4×CH2), 1.94–2.02 (m, 2H, NCH2CH2), 4.69 (dd, 2H, J = 4 Hz, 8 Hz, NCH2), 7.23 (t, 0.5H, J = 8 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.62 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.89 (dd, 1.5H, J = 4 Hz, 8 Hz, Ar–H), 8.16 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 8 Hz, Ar–H), 8.50 (s, 0.75H, H–C=N), 8.53 (d, 1.5H, J = 4 Hz, Ar–H), 9.25 (d, 0.5H, J = 8 Hz, Ar–H), 9.33 (d, 1.5H, J = 8 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 12.67 (CH3), 20.71, 24.11, 24.16, 26.08, 29.31, 29.44, 29.75, 29.78 (5×CH2), 59.71, 59.78 (NCH2), 114.54, 114.76, 114.98, 124.94, 125.90, 128.22, 128.51, 128.59, 128.85, 129.01, 129.04, 143.85, 144.46, 146.19, 148.09 (Ar–C), 157.59, 161.06, 163.53, 164.04 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = −73.48 (s, 3F, CF3), (−109.91 to −109.83), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 455.90 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-heptylpyridinium nitrate (39). It was obtained as yellow crystals; mp: 204–205 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.85–0.89 (m, 3H, CH3), 1.24–1.32 (m, 8H, 4×CH2), 1.94–2.01 (m, 2H, NCH2CH2), 4.70 (t, 2H, J = 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 Hz, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.90 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 8.17 (s, 0.25H, H–C=N), 8.41 (d, 0.5H, J = 8 Hz, Ar–H), 8.52 (s, 0.75H, H–C=N), 8.55 (d, 1.5H, J = 8 Hz, Ar–H), 9.27 (d, 0.5H, J = 4 Hz, Ar–H), 9.36 (d, 1.5H, J = 4 Hz, Ar–H), 12.50 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 14.08 (CH3), 22.12, 25.51, 25.56, 28.20,30.72, 30.84, 31.16, 31.18 (5×CH2), 61.12, 61.18 (NCH2), 115.95, 116.16, 116.37, 126.34, 127.30, 129.57, 129.65, 129.93, 130.01, 130.21, 130.39, 130.42, 145.25, 145.88, 147.47, 149.51, 149.80 (Ar–C), 158.95, 162.12, 162.46, 164.93, 165.42 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.93 to −109.85), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 404.90 [M+].
4-(2-(4-Fluorobenzylidene)hydrazinecarbonyl)-1-heptylpyridinium thiocyanate (40). It was obtained as yellow crystals; mp: 175–176 °C. 1H NMR (400 MHz, DMSO-d6): δH = 0.85–0.89 (m, 3H, CH3), 1.25–1.32 (m, 8H, 4×CH2), 1.94–2.01 (m, 2H, NCH2CH2), 4.70 (t, 2H, J = 8 Hz, NCH2), 7.26 (dd, 0.5H, J = 8 Hz, 12 H, Ar–H), 7.38 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 7.63 (dd, 0.5H, J = 4 Hz, 8 Hz, Ar–H), 7.90 (dd, 1.5H, J = 8 Hz, 12 Hz, Ar–H), 8.17 (s, 0.25H, H–C=N), 8.40 (d, 0.5H, J = 4 Hz, Ar–H), 8.51 (s, 0.75H, H–C=N), 8.54 (d, 1.5H, J = 4 Hz, Ar–H), 9.27 (d, 0.5H, J = 8 Hz, Ar–H), 9.35 (d, 1.5H, J = 4 Hz, Ar–H), 12.51 (bs, 1H, CONH). 13C NMR (100 MHz, DMSO-d6): δC = 14.02 (CH3), 22.07, 25.47, 28.52, 28.16, 30.69, 30.81, 31.11, 31.14 (5×CH2), 61.09, 61.17 (NCH2), 115.89, 116.10, 116.32, 126.30, 127.27, 129.51, 129.60, 129.88, 129.97, 130.16, 130.19, 130.34, 130.37, 145.21, 145.82, 147.42, 149.48, 149.75 (Ar–C), 158.91, 162.08, 162.41, 164.55, 164.89, 165.36 (C=N, C=O). 19F NMR (377 MHz, DMSO-d6): δF = (−109.93 to −109.85), (−109.43 to −109.35) (2m, 1F, Ar–F). MS (ES) m/z = 400.00 [M+].