Aquaporin-11 (AQP11) Expression in the Mouse Brain
Abstract
:1. Introduction
2. Results
2.1. Expression of AQP11 mRNA and Protein in the Mouse Brain
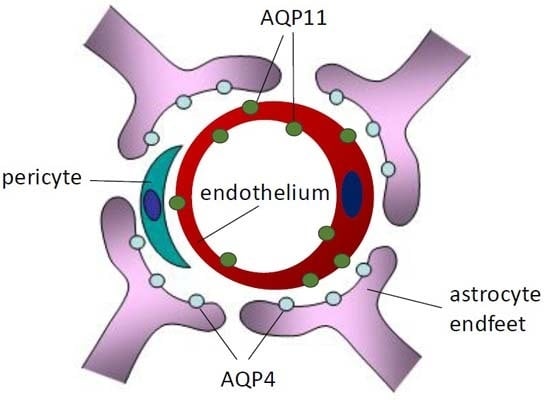
2.2. Localization of AQP11 in the Brain
2.3. The Expression of AQP11 in the Developing Brain
2.4. The Expression of AQP1 and AQP4 mRNA in the Brain of AQP11-Deficient Mice
2.5. The BBB Permeability of AQP11-Deficient Mice Was Normal
3. Discussion
4. Experimental Section
4.1. Animals
4.2. RNA Isolation and Quantitative RT-PCR
4.3. Immunoblotting of AQP11 in the Brain
4.4. Immunohistochemistry
4.5. Biotin Permeability Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carbrey, J.M.; Agre, P. Discovery of the aquaporins and development of the field. Handb. Exp. Pharmacol. 2009, 190, 3–28. [Google Scholar] [PubMed]
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol. Asp. Med. 2012, 33, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. Aquaporin subfamily with unusual NPA boxes. Biochim. Biophys. Acta Biomembr. 2006, 1758, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev. Physiol. Biochem. Pharmacol. 2005, 155, 31–55. [Google Scholar] [PubMed]
- Morishita, Y.; Matsuzaki, T.; Hara-Chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of Aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.H.; Uchida, S.; Sasaki, S.; Ishibashi, K. Identification of a novel Aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sohara, E.; Kobayashi, K.; Chiga, M.; Rai, T.; Ishibashi, K.; Horie, S.; Su, X.; Zhou, J.; Sasaki, S.; et al. Aberrant Glycosylation and Localization of Polycystin-1 Cause Polycystic Kidney in an AQP11 Knockout Model. J. Am. Soc. Nephrol. 2014, 25, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Fukuda, A.M.; Jullienne, A.; Petry, K.G. Aquaporin and brain diseases. Biochim. Biophys. Acta 2014, 1840, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Tait, M.J.; Reza, A.; Davies, D.C.; Bell, B.A.; Verkman, A.S.; Papadopoulos, M.C. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience 2009, 161, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, G.; Kaymaz, F.; Gursoy-Özdemir, Y.; Akalan, N.; Akdemir, E.S. The time course changes in expression of Aquaporin 4 and aquaporin 1 following global cerebral ischemic edema in rat. Surg. Neurol. Int. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ohta, E.; Itoh, T.; Nemoto, T.; Kumagai, J.; Ko, S.B.H.; Ishibashi, K.; Ohno, M.; Uchida, K.; Ohta, A.; Sohara, E.; et al. Pancreas-specific Aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am. J. Physiol. Cell Physiol. 2009, 297, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Tchekneva, E.E.; Khuchua, Z.; Davis, L.S.; Kadkina, V.; Dunn, S.R.; Bachman, S.; Ishibashi, K.; Rinchik, E.M.; Harris, R.C.; Dikov, M.M.; et al. Single amino acid substitution in Aquaporin 11 causes renal failure. J. Am. Soc. Nephrol. 2008, 19, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Füchtbauer, E.-M.; Füchtbauer, A.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific Aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Zammit, C.; di Giovanni, G.; Muscat, R.; Valentino, M. The central role of Aquaporins in the pathophysiology of ischemic stroke. Front. Cell. Neurosci. 2015, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, S.; Tanaka, Y.; Matsuzaki, T.; Morishita, Y.; Ishibashi, K. Aquaporin-11 (AQP11) Expression in the Mouse Brain. Int. J. Mol. Sci. 2016, 17, 861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060861
Koike S, Tanaka Y, Matsuzaki T, Morishita Y, Ishibashi K. Aquaporin-11 (AQP11) Expression in the Mouse Brain. International Journal of Molecular Sciences. 2016; 17(6):861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060861
Chicago/Turabian StyleKoike, Shin, Yasuko Tanaka, Toshiyuki Matsuzaki, Yoshiyuki Morishita, and Kenichi Ishibashi. 2016. "Aquaporin-11 (AQP11) Expression in the Mouse Brain" International Journal of Molecular Sciences 17, no. 6: 861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060861