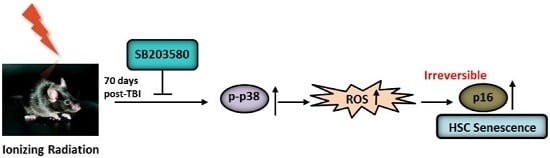
p38 MAPK Inhibitor Insufficiently Attenuates HSC Senescence Administered Long-Term after 6 Gy Total Body Irradiation in Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of SB203580 on Peripheral Blood Cells after TBI
2.2. Effects of SB203580 on BMNC Counts and CFU-GM after TBI
2.3. Effects of SB203580 on TBI-Induced Long-Term HSC Injury
2.4. SB203580 Inhibits TBI-Induced Chronic Oxidative Stress
2.5. SB203580 Inhibits p38 Expression Augment in HSCs but Not Senescence in HSCs
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Total-Body Irradiation (TBI) and SB203580 Treatment
4.3. Peripheral Blood Cell and BM Nucleated Cell (BMNC) Counts
4.4. Colony-Forming Cell (CFC) Assay
4.5. Cobblestone Area-Forming Cell (CAFC) and Single-Cell Colony Assays
4.6. Analysis of the Levels of Intracellular Reactive Oxygen Species (ROS) via Flow Cytometry
4.7. Immunofluorescence Staining for p-p38 and p16
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munoz-Espin, D.; Canamero, M.; Maraver, A.; Gomez-Lopez, G.; Contreras, J.; Murillo-Cuesta, S.; Rodriguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Wang, Y.; Brown, S.A.; van Zant, G.; Zhou, D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp. Hematol. 2003, 31, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Wang, Y.; van Zant, G.; Zhou, D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003, 63, 5414–5419. [Google Scholar] [PubMed]
- Li, C.; Lu, L.; Zhang, J.; Huang, S.; Xing, Y.; Zhao, M.; Zhou, D.; Li, D.; Meng, A. Granulocyte colony-stimulating factor exacerbates hematopoietic stem cell injury after irradiation. Cell Biosci. 2015, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Zhou, D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat. Res. 2011, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kellner, J.; Liu, L.; Zhou, D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011, 20, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. P38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014, 20, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Testa, N.G.; Hendry, J.H.; Molineux, G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985, 5, 101–110. [Google Scholar] [PubMed]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Wu, H.; Lu, L.; Wang, X.; Zhang, J.; Zhang, H.; Fan, S.; Fan, F.; Zhou, D.; et al. The effects of p38 MAPK inhibition combined with g-csf administration on the hematoimmune system in mice with irradiation injury. PLoS ONE 2013, 8, e62921. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, H.; Pazhanisamy, S.K.; Meng, A.; Wang, Y.; Zhou, D. Reactive oxygen species and hematopoietic stem cell senescence. Int. J. Hematol. 2011, 94, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wu, H.; Zhang, J.; Li, D.; Wang, Y.; Wang, Y.; Zhang, H.; Lu, L.; Li, C.; Huang, S.; et al. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2015, 87, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 mapk to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Popova, N.R.; Bruskov, V.I. Radioprotectors: History, trends and prospects. Biofizika 2015, 60, 801–811. [Google Scholar] [PubMed]
- Li, D.; Wang, Y.; Wu, H.; Lu, L.; Zhang, H.; Chang, J.; Zhai, Z.; Zhang, J.; Wang, Y.; Zhou, D.; et al. Mitigation of ionizing radiation-induced bone marrow suppression by p38 inhibition and g-csf administration. J. Radiat. Res. 2011, 52, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ema, H.; Morita, Y.; Yamazaki, S.; Matsubara, A.; Seita, J.; Tadokoro, Y.; Kondo, H.; Takano, H.; Nakauchi, H. Adult mouse hematopoietic stem cells: Purification and single-cell assays. Nat. Protoc. 2006, 1, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Feng, W.; Li, H.; Gardner, D.; Luo, Y.; Wang, Y.; Liu, L.; Meng, A.; Sharpless, N.E.; Zhou, D. Total body irradiation causes long-term mouse bm injury via induction of hsc premature senescence in an ink4a- and arf-independent manner. Blood 2014, 123, 3105–3115. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Wang, Y.-Y.; Zhang, J.-L.; Li, D.-G.; Meng, A.-M. p38 MAPK Inhibitor Insufficiently Attenuates HSC Senescence Administered Long-Term after 6 Gy Total Body Irradiation in Mice. Int. J. Mol. Sci. 2016, 17, 905. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060905
Lu L, Wang Y-Y, Zhang J-L, Li D-G, Meng A-M. p38 MAPK Inhibitor Insufficiently Attenuates HSC Senescence Administered Long-Term after 6 Gy Total Body Irradiation in Mice. International Journal of Molecular Sciences. 2016; 17(6):905. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060905
Chicago/Turabian StyleLu, Lu, Yue-Ying Wang, Jun-Ling Zhang, De-Guan Li, and Ai-Min Meng. 2016. "p38 MAPK Inhibitor Insufficiently Attenuates HSC Senescence Administered Long-Term after 6 Gy Total Body Irradiation in Mice" International Journal of Molecular Sciences 17, no. 6: 905. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060905