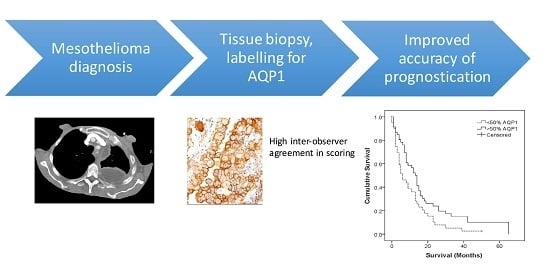
Usefulness of Aquaporin 1 as a Prognostic Marker in a Prospective Cohort of Malignant Mesotheliomas
Abstract
:1. Introduction
2. Results
2.1. Immunohistochemical Analysis
2.2. AQP1 as a Prognostic Indicator
2.3. Established Prognostic Indicators
2.4. Reproducibility of AQP Scoring in Histological Sections
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Immunohistochemical Analysis
4.3. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gatta, G.; Ciccolallo, L.; Kunkler, I.; Capocaccia, R.; Berrino, F.; Coleman, M.P.; de Angelis, R.; Faivre, J.; Lutz, J.M.; Martinez, C.; et al. Survival from rare cancer in adults: A population-based study. Lancet Oncol. 2006, 7, 132–140. [Google Scholar] [CrossRef]
- Henderson, D.W.; Reid, G.; Kao, S.C.; van Zandwijk, N.; Klebe, S. Challenges and controversies in the diagnosis of malignant mesothelioma: Part 2. Malignant mesothelioma subtypes, pleural synovial sarcoma, molecular and prognostic aspects of mesothelioma, BAP1, aquaporin-1 and microRNA. J. Clin. Pathol. 2013, 66, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.; Tabata, C.; Tabata, R.; Maeda, R.; Yasumitsu, A.; Yamada, S.; Kuribayashi, K.; Fukuoka, K.; Nakano, T. Pleural effusion VEGF levels as a prognostic factor of malignant pleural mesothelioma. Respir. Med. 2011, 105, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.E.; Elvers, K.T.; Welsh, G.I.; Millar, A.B.; Maskell, N.A. VEGF and sVEGFR-1 in malignant pleural effusions: Association with survival and pleurodesis outcomes. Lung Cancer 2012, 77, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.; Harvie, R.; Paturi, F.; Taylor, R.; Davey, R.; Abraham, R.; Clarke, S.; Marx, G.; Cullen, M.; Kerestes, Z.; et al. The predictive role of serum vegf in an advanced malignant mesothelioma patient cohort treated with thalidomide alone or combined with cisplatin/gemcitabine. Lung Cancer 2012, 75, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Shridhar, V.; Bright, R.K.; Kalemkerian, G.P.; Du, W.; Carbone, M.; Watanabe, Y.; Pass, H.I. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br. J. Cancer 1999, 81, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.; Armstrong, N.; Condon, B.; Griggs, K.; McCaughan, B.; Maltby, S.; Wilson, A.; Henderson, D.W.; Klebe, S. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer 2012, 118, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Agre, P. Pathophysiology of the aquaporin water channels. Annu. Rev. Physiol. 1996, 58, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Physiological importance of aquaporin water channels. Ann. Med. 2002, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campos, J.L.; Sanchez Silva, R.; Gomez Izquierdo, L.; Marquez, E.; Ortega Ruiz, F.; Cejudo, P.; Barrot Cortes, E.; Toledo Aral, J.J.; Echevarria, M. Overexpression of aquaporin-1 in lung adenocarcinomas and pleural mesotheliomas. Histol. Histopathol. 2011, 26, 451–459. [Google Scholar] [PubMed]
- Nicchia, G.; Stigliano, C.; Sparaneo, A.; Rossi, A.; Frigeri, A.; Svelto, M. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 2013, 91, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Klebe, S.; Griggs, K.; Cheng, Y.; Driml, J.; Henderson, D.W.; Reid, G. Blockade of aquaporin 1 inhibits proliferation, motility, and metastatic potential of mesothelioma in vitro but not in an in vivo model. Dis. Markers 2015, 2015, 286719. [Google Scholar] [CrossRef] [PubMed]
- Organising Committee. Guidelines for the Diagnosis and Treatment of Malignant Pleural Mesothelioma; ADRI: Sydney, Ausralia, 2013; p. 39. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, J.; Bai, C. Role of aquaporin and sodium channel in pleural water movement. Respir. Physiol. Neurobiol. 2003, 139, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Imredi, E.; Toth, B.; Doma, V.; Barbai, T.; Raso, E.; Kenessey, I.; Timar, J. Aquaporin 1 protein expression is associated with BRAF V600 mutation and adverse prognosis in cutaneous melanoma. Melanoma Res. 2016, 26, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lehnerdt, G.F.; Bachmann, H.S.; Adamzik, M.; Panic, A.; Koksal, E.; Weller, P.; Lang, S.; Schmid, K.W.; Siffert, W.; Bankfalvi, A. AQP1, AQP5, Bcl-2 and p16 in pharyngeal squamous cell carcinoma. J. Laryngol. Otol. 2015, 129, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.Y.; Ding, D.G. Expression of aquaporin 1 in bladder uroepithelial cell carcinoma and its relevance to recurrence. Asian Pac. J. Cancer Prev. 2015, 16, 3973–3976. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, H.; Shao, Y.; Liu, X.; Yang, L.; Huang, Y.; Fu, L.; Gu, F.; Ma, Y. Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients. Oncotarget 2016, 7, 8143–8154. [Google Scholar] [PubMed]
- Wu, Z.; Li, S.; Liu, J.; Shi, Y.; Wang, J.; Chen, D.; Luo, L.; Qian, Y.; Huang, X.; Wang, H. Rnai-mediated silencing of aqp1 expression inhibited the proliferation, invasion and tumorigenesis of osteosarcoma cells. Cancer Biol. Ther. 2015, 16, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, R.M.; Apostolidou, E.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C.; Zarogiannis, S.G. Influence of AQP1 on cell adhesion, migration and tumor sphere formation in malignant pleural mesothelioma is substratum and histological type dependent. Am. J. Physiol. 2016, 310, L489–L495. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; de la Escalera, G.M. Aquaporin-1: A novel promoter of tumor angiogenesis. Trend Endocrinol. Metab. 2006, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- El Hindy, N.; Bankfalvi, A.; Herring, A.; Adamzik, M.; Lambertz, N.; Zhu, Y.; Siffert, W.; Sure, U.; Sandalcioglu, I.E. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013, 33, 609–613. [Google Scholar] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflugers Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Galateau-Salle, F.; Churg, A.; Roggli, V.; Travis, W.D. The 2015 world health organization classification of tumors of the pleura: Advances since the 2004 classification. J. Thorac. Oncol. 2016, 11, 142–154. [Google Scholar] [CrossRef] [PubMed]

| Variable | Count (%) | |
|---|---|---|
| Age in Years, Median (Range) | 71 (40–91) | |
| Sex | Male | 71 (78) |
| Female | 20 (22) | |
| Type | Pleural | 85 (93) |
| Peritoneal | 6 (7) | |
| Subtype | Epithelioid | 66 (73) |
| Biphasic | 11 (12) | |
| Sarcomatoid | 14 (15) | |
| Treatment (Some patients had 2 treatment modalities) | Radical surgery (EPP) | 1 (1) |
| Chemotherapy | 17 (19) | |
| Radiotherapy | 6 (7) | |
| Conservative | 71 (76) | |
| AQP1 Score | <50% | 47 (52) |
| ≥50% | 44 (48) | |
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR | p | HR | p | ||
| Age | <71 | 1.0 | 1.0 | 0.007 | |
| >71 | 1.754 | 1.893 | |||
| Sex | Female | 1.0 | 1.0 | 0.813 | |
| Male | 1.011 | 0.968 | 1.061 | ||
| Subtype | Epithelioid | 1.0 | 1.0 | ||
| Sarcomatoid | 2.757 | 0.003 | 2.633 | 0.014 | |
| Biphasic | 2.048 | 0.022 | 1.752 | 0.099 | |
| AQP1 Status | <50% | 1.830 | 0.008 | 1.367 | 0.233 |
| >50% | 1.0 | 1.0 | |||
| AQP1 Score | Pleural | Peritoneal |
|---|---|---|
| <50% | 43 | 4 |
| ≥50% | 51 | 2 |
| AQP1 Score | Epithelioid | Biphasic | Sarcomatoid |
|---|---|---|---|
| <50% | 22 | 12 | 13 |
| ≥50% | 50 | 2 | 1 |
| Survival Statistic | Epithelioid | Biphasic | Sarcomatoid |
|---|---|---|---|
| Mean | 16.7 | 7.6 | 6.2 |
| Median | 13.5 | 4.5 | 2.0 |
| Minimum | 0 | 0 | 0 |
| Maximum | 65 | 24 | 39 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driml, J.; Pulford, E.; Moffat, D.; Karapetis, C.; Kao, S.; Griggs, K.; Henderson, D.W.; Klebe, S. Usefulness of Aquaporin 1 as a Prognostic Marker in a Prospective Cohort of Malignant Mesotheliomas. Int. J. Mol. Sci. 2016, 17, 1041. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071041
Driml J, Pulford E, Moffat D, Karapetis C, Kao S, Griggs K, Henderson DW, Klebe S. Usefulness of Aquaporin 1 as a Prognostic Marker in a Prospective Cohort of Malignant Mesotheliomas. International Journal of Molecular Sciences. 2016; 17(7):1041. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071041
Chicago/Turabian StyleDriml, Jack, Emily Pulford, David Moffat, Christos Karapetis, Steven Kao, Kim Griggs, Douglas Warrington Henderson, and Sonja Klebe. 2016. "Usefulness of Aquaporin 1 as a Prognostic Marker in a Prospective Cohort of Malignant Mesotheliomas" International Journal of Molecular Sciences 17, no. 7: 1041. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071041