The Salicylic Acid-Mediated Release of Plant Volatiles Affects the Host Choice of Bemisia tabaci
Abstract
:1. Introduction
2. Results
2.1. Establishment of Nonviruliferous and Viruliferous B. tabaci Colonies
2.2. Choice Test of B. tabaci on SA-Treated vs. Control Plants
2.3. Extraction and Analysis of Volatiles
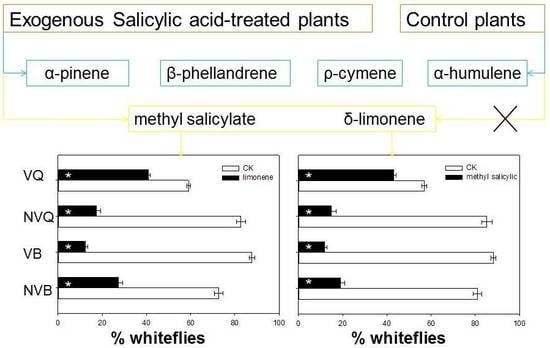
2.4. Effects of Methyl Salicylate and δ-Limonene on Whitefly Behavior as Determined with a Y-Tube Olfactometer
3. Discussion
4. Materials and Methods
4.1. Host Plants
4.2. Establishment of Nonviruliferous and Viruliferous B. tabaci Colonies
4.3. Effect of Salicylic Acid (SA) Application on Tomato Plants
4.4. Choice Test of B. tabaci on SA-Treated vs. Control Plants
4.5. Extraction and Analysis of Volatiles
4.6. Y-Tube Olfactometer Assays
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Pan, H.P.; Chu, D.; Liu, B.M.; Shi, X.B.; Guo, L.T.; Xie, W.; Carrière, Y.; Li, X.C.; Zhang, Y.J. Differential effects of an exotic plant virus on its two closely related vectors. Sci. Rep. 2013, 3, 2230. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.P.; Chu, D.; Yan, W.Q.; Su, Q.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Li, R.M. Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 2012, 7, e34817. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Preisser, E.L.; Chu, D.; Pan, H.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; Zhou, X.G.; Zhang, Y.J. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and tomato yellow leaf curl virus. J. Virol. 2013, 87, 4929. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pan, H.P.; Xie, W.; Wang, S.L.; Wu, Q.J.; Fang, Y.; Shi, X.B.; Zhang, Y.J. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 2013, 3, 2253. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.W.; Tumlinson, J.H. Plant volatiles as defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Preston, C.A. The eco-physiological complexity of plant responses to insect herbivores. Planta 1999, 208, 137–145. [Google Scholar] [CrossRef]
- Kahl, J.; Siemens, D.H.; Aerts, R.J.; Gabler, R.; Kuhnemann, F.; Preston, C.A.; Baldwin, I.T. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 2000, 210, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- VanDoorn, A.; de Vries, M.; Kant, M.R.; Schuurink, R.C. Whiteflies glycosylate salicylic acid and secrete the conjugate via their honeydew. J. Chem. Ecol. 2015, 41, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.B.; Pan, H.P.; Xie, W.; Wu, Q.J.; Wang, S.L.; Liu, Y.; Fang, Y.; Chen, G.; Gao, X.W.; Zhang, Y.J. Plant virus differentially alters the plant’s defense response to its closely related vectors. PLoS ONE 2013, 8, e83520. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Agrawal, A.A.; Halitschke, R. Salicylate-mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. Adaptive defense responses to pathogens and insects. Adv. Bot. Res. 2009, 51, 551–612. [Google Scholar]
- Mohasea, L.; van der Westhuizen, A.J. Salicylic acid is involved in resistance responses in the Russian wheat aphid-wheat interaction. J. Plant Physiol. 2002, 159, 585–590. [Google Scholar] [CrossRef]
- Heidel, A.J.; Baldwin, I.T. Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ. 2004, 27, 1362–1373. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Song, G.C.; Ryu, C.M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [PubMed]
- Schütz, S.; Weißbecker, B.; Klein, A.; Hummel, H.E. Host plant selection of the Colorado potato beetle as influenced by damage induced volatiles of the potato plant. Naturwissenschaften 1997, 84, 212–217. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Van Poecke, R.M.P.; Dicke, M. Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J. Exp. Bot. 2002, 53, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Kappers, I.F.; Aharoni, A.; van Herpen, T.W.; Luckerhoff, L.L.P.; Dicke, M.; Bouwmeester, H.J. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 2005, 309, 2070–2072. [Google Scholar] [CrossRef] [PubMed]
- Gols, R.; Roosjen, M.; Dijkman, H.; Dicke, M. Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid and spider mite infestation. J. Chem. Ecol. 2003, 29, 2651–2666. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Ren, S.L.; Xue, X.; Ren, S.X.; Cuthbertson, A.G.; van Dam, N.M.; Qiu, B.L. Efficiency of plant induced volatiles in attracting Encarsia formosa and Serangium japonicum, two dominant natural enemies of whitefly Bemisia tabaci in China. Pest Manag. Sci. 2014, 70, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zeng, J.; Zhong, G. The push-pull strategy for citrus psyllid control. Pest Manag. Sci. 2015, 71, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Brunissen, L.; Vincent, C.; Roux, V.L.; Giordanengo, P. Effects of systemic potato response to wounding and jasmonate on the aphid Macrosiphum euphorbiae (Sternorryncha: Aphididae). J. Appl. Entomol. 2010, 134, 562–571. [Google Scholar]
- Girling, R.D.; Hassall, M.; Turner, J.G.; Poppy, G.M. Behavioural responses of the aphid parasitoid Diaeretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol. Exp. Appl. 2006, 120, 1–9. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Karb, M.J. Plasticity in allocation of nicotine to reproductive parts in Nicotiana attenuata. J. Chem. Ecol. 1995, 21, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.R.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, D.F.; Brown, G.C.; Jackson, D.M.; Hamiltonkemp, T.R. Effects of some leaf-emitted volatile compounds on aphid population increase. J. Chem. Ecol. 1993, 19, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared signals—“Alarm calls” from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Matsui, K.; Takabayashi, J. Chemical and molecular ecology of herbivore-induced pant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, J.; Arimura, G.; Ozawa, R.; Shimoda, T.; Takabayashi, J.; Nishioka, T. A comparison of the response of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2003, 38, 109–116. [Google Scholar]
- Saad, K.A.; Mohamad Roff, M.N.; Hallett, R.H.; Idris, A.B. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, K.; Karban, R. Plant age, communication, and resistance to herbivores: Young sagebrush plants are better emitters and receivers. Oecologia 2006, 149, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Cañas, L.A. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J. Chem. Ecol. 2003, 29, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Gase, K.; Hui, D.; Schmidt, D.D.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol. 2003, 131, 1894–1902. [Google Scholar] [PubMed]
- Will, T.; van Bel, A.J. Induction as well as suppression: How aphid saliva may exert opposite effects on plant defense. Plant Signal. Behav. 2008, 3, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Sugimoto, K.; Ramadan, A.; Arimura, G. Memory of plant communications for priming anti-herbivore responses. Sci. Rep. 2013, 3, 1872. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Andrés, V.S.; Cervera, M.; Redondo, A.; Alquézar, B.; Shimada, T.; Gadea, J.; Rodrigo, M.J.; Zacarías, L.; Palou, L.; et al. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011, 156, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Induced synthesis of plant volatiles. Nature 1997, 385, 30–31. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Lengwiler, U.B.; Bernasconi, M.L.; Wechsler, D. Timing of induced volatile emissions in maize seedlings. Planta 1998, 207, 146–152. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Papachristos, D.P.; Papadopoulos, N.T. Are citrus species favorable hosts for the Mediterranean fruit fly? A demographic perspective. Entomol. Exp. Appl. 2009, 132, 1–12. [Google Scholar] [CrossRef]
- Park, S.W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, A.; Peters, P.; Brückner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Mattarozzi, M.; Musci, M.; Concina, I.; Falasconi, M.; Gobbi, E.; Pardo, M.; Sberveglieri, G. Differentiation of the volatile profile of microbiologically contaminated canned tomatoes by dynamic headspace extraction followe d by gas chromatography-mass spectrometry analysis. Talanta 2009, 77, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Paré, P.W. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 2002, 61, 545–554. [Google Scholar] [CrossRef]
- Takayama, K.; Jansen, R.M.C.; van Henten, E.J.; Verstappen, F.W.A.; Bouwmeester, H.J.; Nishina, H. Emission index for evaluation of volatile organic compounds emitted from tomato plants in greenhouses. Biosyst. Eng. 2012, 113, 220–228. [Google Scholar] [CrossRef]
- Degenhardt, D.C.; Refi-Hind, S.; Stratmann, J.W.; Lincoln, D.E. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 2010, 71, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Dai, F.M.; Zhou, X.P. First report of tomato yellow leaf curl virus in China. Ann. Appl. Biol. 2006, 155, 439–448. [Google Scholar]
- Chu, D.; Wan, F.H.; Zhang, Y.J.; Brown, J.K. Change in the biotype composition of Bemisia tabaci in shandong province of China from 2005 to 2008. Environ. Entomol. 2010, 39, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.J.; Broekgaarden, C.; Zheng, S.J.; Snoeren, T.A.L.; van Loon, J.J.A.; Gols, R.; Dicke, M. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol. 2013, 197, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef] [PubMed]



| Volatile Compound | Control Plants b | SA-Treated Plants c |
|---|---|---|
| 3-Hexenal | 0.422 ± 0.112 A | 0.561 ± 0.098 A |
| 3-Hexen-1-ol | 0.130 ± 0.012 A | 0.376 ± 0.070 A |
| α-Pinene | 0.029 ± 0.007 A | 0.245 ± 0.027 B |
| β-Myrcene | 0.819 ± 0.197 A | 1.826 ± 0.864 A |
| β-Phellandrene | 0.089 ± 0.037 A | 0.513 ± 0.099 B |
| β-Caryophyllene | 0.939 ± 0.303 A | 1.612 ± 0.072 A |
| ρ-Cymene | 0.325 ± 0.069 A | 1.188 ± 0.092 B |
| α-Phellandrene | 2.935 ± 0.096 A | 2.485 ± 0.151 A |
| α-Humulene | 0.073 ± 0.020 A | 0.307 ± 0.037 B |
| Methyl salicylate | n.d. | 3.428 ± 0.141 |
| δ-Limonene | n.d. | 2.420 ± 0.146 |
| Independent Variable | Dependent Variable a | Independent Variable | Dependent Variable |
|---|---|---|---|
| Methyl salicylate | <0.001 *** | δ-Limonene | <0.001 *** |
| V b | 0.045 * | V | 0.542 |
| Biotype c | 0.884 | Biotype | 0.542 |
| Methyl salicylate × V | <0.001 *** | δ-Limonene × V | 0.225 |
| Methyl salicylate × Biotype | <0.001 *** | δ-Limonene × Biotype | <0.001 *** |
| V × Biotype | <0.001 *** | V × Biotype | 0.189 |
| Methyl salicylate × V × Biotype | <0.001 *** | δ-Limonene × V × Biotype | <0.001 *** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Chen, G.; Tian, L.; Peng, Z.; Xie, W.; Wu, Q.; Wang, S.; Zhou, X.; Zhang, Y. The Salicylic Acid-Mediated Release of Plant Volatiles Affects the Host Choice of Bemisia tabaci. Int. J. Mol. Sci. 2016, 17, 1048. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071048
Shi X, Chen G, Tian L, Peng Z, Xie W, Wu Q, Wang S, Zhou X, Zhang Y. The Salicylic Acid-Mediated Release of Plant Volatiles Affects the Host Choice of Bemisia tabaci. International Journal of Molecular Sciences. 2016; 17(7):1048. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071048
Chicago/Turabian StyleShi, Xiaobin, Gong Chen, Lixia Tian, Zhengke Peng, Wen Xie, Qingjun Wu, Shaoli Wang, Xuguo Zhou, and Youjun Zhang. 2016. "The Salicylic Acid-Mediated Release of Plant Volatiles Affects the Host Choice of Bemisia tabaci" International Journal of Molecular Sciences 17, no. 7: 1048. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071048