Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia
Abstract
:1. Introduction
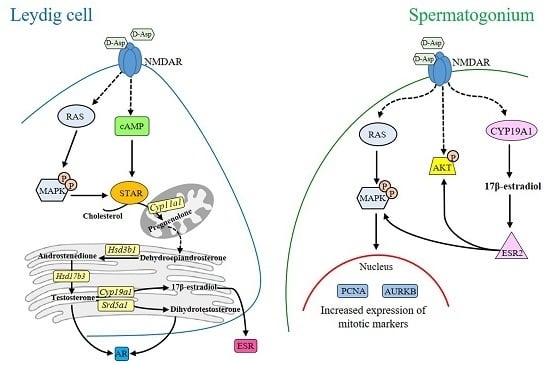
2. d-Asp and Leydig Cells
3. d-Asp and Spermatogonial Proliferation
4. d-Asp and Spermatozoa Maturation
5. Conclusions
Conflicts of Interest
References
- Flück, C.E.; Pandey, A.V. Steroidogenesis of the testis—New genes and pathways. Ann. Endocrinol. 2014, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.J. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 2015, 103, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, M.M.; Santillo, A.; Baccari, G.C. Current knowledge of d-aspartate in glandular tissues. Amino Acids 2014, 46, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Homma, H.; Lee, J.-A.; Fukushima, T.; Santa, T.; Tashiro, K.; Iwatsubo, T.; Imai, K. Localization of d-aspartic acid in elongate spermatids in rat testis. Arch. Biochem. Biophys. 1998, 351, 96–105. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Ronsini, S.; Guida, F.; Spinelli, P.; D’Aniello, A. Occurrence of d-aspartic acid in human seminal plasma and spermatozoa: Possible role in reproduction. Fertil. Steril. 2005, 84, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; di Fiore, M.M. The reproductive activity in the testis of Podarcis s. sicula involves d-aspartic acid: A study on c-kit receptor protein, tyrosine kinase activity and PCNA protein during annual sexual cycle. Gen. Comp. Endocrinol. 2009, 161, 373–383. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, A.; di Fiore, M.M.; D’Aniello, G.; Colin, F.E.; Lewis, G.; Setchell, B.P. Secretion of d-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett. 1998, 436, 23–27. [Google Scholar] [CrossRef]
- D’Aniello, A.; di Cosmo, A.; di Cristo, C.; Annunziato, L.; Petrucelli, L.; Fisher, G. Involvement of d-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996, 59, 97–104. [Google Scholar] [CrossRef]
- D’Aniello, A.; di Fiore, M.M.; Fisher, G.H.; Milone, A.; Seleni, A.; D’Aniello, S.; Perna, A.F.; Ingrosso, D. Occurrence of d-aspartic acid and N-methyl-d-aspartic acid in rat neuroendocrine tissues and their role in the modulation of Luteinizing hormone and Growth hormone release. FASEB J. 2000, 14, 699–714. [Google Scholar] [PubMed]
- D’Aniello, G.; Tolino, A.; D’Aniello, A.; Errico, F.; Fisher, G.H.; di Fiore, M.M. The role of d-aspartic acid and N-Methyl-d-Aspartic acid in the regulation of prolactin release. Endocrinology 2000, 141, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of d-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Chieffi, P.; Burrone, L.; Baccari, G.C.; Longobardi, S.; di Fiore, M.M. d-Aspartate affects NMDA receptor-extracellular signal-regulated kinase pathway and upregulates androgen receptor expression in the rat testis. Theriogenology 2014, 81, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Homma, H.; Lee, J.-A.; Imai, K. d-Aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999, 444, 160–164. [Google Scholar] [CrossRef]
- Nagata, Y.; Homma, H.; Matsumoto, M.; Imai, K. Stimulation of steroidogenic acute regulatory (StAR) gene expression by d-aspartate in rat Leydig cells. FEBS Lett. 1999, 454, 317–320. [Google Scholar] [PubMed]
- Manna, P.R.; Stocco, D.M. Regulation of the steroidogenic acute regulatory protein expression: Functional and physiological consequences. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 93–108. [Google Scholar] [CrossRef]
- Lavoie, H.A.; King, S.R. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp. Biol. Med. 2009, 234, 880–907. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Assisi, L.; D’Aniello, S.; Spinelli, P.; Botte, V.; di Fiore, M.M. Testicular endocrine activity is upregulated by d-aspartic acid in the green frog, Rana esculenta. J. Endocrinol. 2004, 182, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; D’Aniello, S.; di Fiore, M.M. Endocrine roles of d-Aspartic acid in the testis of lizard Podarcis s. sicula. J. Endocrinol. 2005, 187, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; di Fiore, M.M. d-Asp: A new player in reproductive endocrinology of the amphibian Rana esculenta. J. Chromatogr. B 2011, 879, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Burrone, L.; Raucci, F.; di Fiore, M.M. Steroidogenic gene expression following d-aspartate treatment in frog testis. Gen. Comp. Endocrinol. 2012, 175, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Falvo, S.; Chieffi, P.; di Fiore, M.M.; Senese, R.; Chieffi Baccari, G. d-AspartateInduces Proliferative Pathways in Spermatogonial GC-1 Cells. J. Cell. Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.C.; Fagg, G.E. Comparison of l-[3H]glutamate, d-[3H]aspartate, dl-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur. J. Pharmacol. 1987, 133, 291–300. [Google Scholar] [CrossRef]
- Di Giovanni, M.; Topo, E.; Santillo, A.; D’Aniello, A.; Chieffi Baccari, G. d-Aspartate binding sites in rat Harderian gland. Amino Acids 2010, 38, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Errico, F.; Napolitano, F.; Nisticò, R.; Usiello, A. New insights on the role of free d-aspartate in the mammalian brain. Amino Acids 2012, 43, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Shi, T.; Sweedler, J.V. d-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012, 43, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Doherty, F.C.; Sladek, C.D. NMDA receptor subunit expression in the supraoptic nucleus of adult rats: Dominance of NR2B and NR2D. Brain Res. 2011, 1388, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Hinoi, E.; Balcar, V.J.; Taniura, H.; Yoneda, Y. Possible expression of functional glutamate transporters in the rat testis. J. Endocrinol. 2004, 181, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Yang, N.; Ma, Y.H.; Jiang, J.; Zhang, J.F.; Fei, J.; Guo, L.H. Identification of glutamate transporters and receptors in mouse testis. Acta Pharmacol. Sin. 2004, 25, 366–371. [Google Scholar] [PubMed]
- Gill, S.S.; Mueller, R.W.; McGuire, P.F.; Pulido, O.M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol. Pathol. 2000, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Pulido, O.M. Glutamate receptors in peripheral tissues: Current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Chandler, L.J.; Sutton, G.; Dorairaj, N.R.; Norwood, D. N-methyl d-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J. Biol. Chem. 2001, 276, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.H. MAP kinase pathways. Prog. Biophys. Mol. Biol. 1999, 71, 479–500. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Krapivinsky, L.; Manasian, Y.; Ivanov, A.; Tyzio, R.; Pellegrino, C.; Ben-Ari, Y.; Clapham, D.E.; Medina, I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 2003, 40, 775–784. [Google Scholar] [CrossRef]
- Pathirana, I.N.; Kawate, N.; Büllesbach, E.E.; Takahashi, M.; Hatoya, S.; Inaba, T.; Tamada, H. Insulin-like peptide 3 stimulates testosterone secretion in mouse Leydig cells via cAMP pathway. Regul. Pept. 2012, 178, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Andric, S.A.; Janjic, M.M.; Stojkov, N.J.; Kostic, T.S. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E544–E550. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; D’Aniello, A.; di Fiore, M.M. Stimulation of androgen production by d-aspartate through the enhancement of StAR, P450scc and 3β-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells. Steroids 2014, 84, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Furuchi, T.; Homma, H. Free d-aspartate in mammals. Biol. Pharm. Bull. 2005, 28, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; de Toni, L.; Ferigo, M.; Rocca, M.S.; Speltra, E.; Ferlin, A.; Foresta, C. d-Aspartic acid stimulates steroidogenesis through the delay of LH receptor internalization in a mammalian Leydig cell line. J. Endocrinol. Investig. 2016, 39, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Assisi, L.; Botte, V.; di Fiore, M.M. Endogenous testicular d-aspartic acid regulates gonadal aromatase activity in boar. J. Endocrinol. Investig. 2006, 29, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Assisi, L.; Botte, V.; di Fiore, M.M. Involvement of d-Asp in P450 aromatase activity and estrogen receptors in boar testis. Amino Acids 2007, 32, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Burrone, L.; Santillo, A.; Pinelli, C.; Chieffi Baccari, G.; di Fiore, M.M. Induced synthesis of P450 aromatase and 17β-estradiol by d-aspartate in frog brain. J. Exp. Biol. 2012, 215, 3559–3565. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Pinelli, C.; Burrone, L.; Baccari, G.C.; di Fiore, M.M. d-Aspartic acid implication in the modulation of frog brain sex steroid levels. Gen. Comp. Endocrinol. 2013, 181, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Assisi, L.; Botte, V.; D’Aniello, A.; di Fiore, M.M. Enhancement of aromatase activity by d-aspartic acid in the ovary of the lizard Podarcis s. sicula. Reproduction 2001, 121, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, P.; Giordano, A. Following the tracks of AKT1 gene. Cancer Biol. Ther. 2007, 6, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.; Colucci-D’Amato, G.L.; Guarino, F.; Salvatore, G.; Angelini, F. 17β-estradiol induces spermatogonial proliferation through mitogen-activated protein kinase (extracellular signal-regulated kinase 1) activity in the lizard (Podarcis s. sicula). Mol. Reprod. Dev. 2002, 61, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.; Chieffi, S. Molecular biomarkers as potential targets for therapeutic strategies in human testicular germ cell tumors: An overview. J. Cell. Physiol. 2013, 228, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Namekawa, S.H.; Saga, Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells 2013, 31, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Beach, D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell 1993, 4, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M.; Earnshaw, W.C. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Libertini, S.; Franco, R.; Abagnale, A.; Marra, L.; Portella, G.; Chieffi, P. Aurora B expression in post-puberal testicular germ cell tumours. J. Cell. Physiol. 2009, 221, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Portella, G.; Passaro, C.; Chieffi, P. Aurora B: A new prognostic marker and therapeutic target in cancer. Curr. Med. Chem. 2011, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Castoria, G.; Migliaccio, A.; Bilancio, A.; di Domenico, M.; de Falco, A.; Lombardi, M.; Fiorentino, R.; Varricchio, L.; Barone, M.V.; Auricchio, F. PI3-kinase in concert with Srcpromotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001, 20, 6050–6059. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Tanaka, H.; Kageyama, S.; Nagasawa, M.; Wada, A.; Murai, R.; Kobayashi, K.; Hanada, E.; Agata, Y.; Kawauchi, A. The Effect of d-Aspartate on Spermatogenesis in Mouse Testis. Biol. Reprod. 2016, 94, 30. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Bunick, D.; Hess, R.A.; Janulis, L.; Newton, S.C.; Millette, C.F.; Osawa, Y.; Shizuta, Y.; Toda, K.; Bahr, J.M. Germ cells of the mouse testis express P450 aromatase. Endocrinology 1993, 132, 1396–1401. [Google Scholar] [PubMed]
- Carreau, S.; Bouraima-Lelong, H.; Delalande, C. Estrogens: New players in spermatogenesis. Reprod. Biol. 2011, 11, 174–193. [Google Scholar] [CrossRef]
- Carpino, A.; Pezzi, V.; Rago, V.; Bilinska, B.; Andò, S. Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell 2001, 33, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Castoria, G.; di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Bottero, D.; Varricchio, L.; Nanayakkara, M.; Rotondi, A.; et al. Sex steroid hormones act as growth factors. J. Steroid Biochem. Mol. Biol. 2002, 83, 31–35. [Google Scholar] [CrossRef]
- Vicini, E.; Loiarro, M.; di Agostino, S.; Corallini, S.; Capolunghi, F.; Carsetti, R.; Chieffi, P.; Geremia, R.; Stefanini, M.; Sette, C. 17β-estradiol elicits genomic and non-genomic responses in mouse male germ cells. J. Cell. Physiol. 2006, 206, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Chimento, A.; Ruggiero, C.; de Luca, A.; Lappano, R.; Andò, S.; Maggiolini, M.; Pezzi, V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17β-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 2008, 149, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Falvo, S.; di Fiore, M.M.; Burrone, L.; Chieffi Baccari, G.; Longobardi, S.; Santillo, A. Androgen and oestrogen modulation by d-aspartate in rat epididymis. Reprod. Fertil. Dev. 2016, in press. [Google Scholar]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hamzeh, M. Androgen action in the epididymis. J. Androl. 2011, 32, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Shur, B.D.; Hess, R.A. Estrogen, efferent ductules, and the epididymis. Biol. Reprod. 2011, 84, 207–217. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Ronsini, S.; Notari, T.; Grieco, N.; Infante, V.; D’Angelo, N.; Mascia, F.; di Fiore, M.M.; Fisher, G.; D’Aniello, A. d-Aspartate, a Key Element for the Improvement of Sperm Quality. Adv. Sex. Med. 2012, 2, 47–53. [Google Scholar] [CrossRef]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fiore, M.M.; Santillo, A.; Falvo, S.; Longobardi, S.; Chieffi Baccari, G. Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia. Int. J. Mol. Sci. 2016, 17, 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071127
Di Fiore MM, Santillo A, Falvo S, Longobardi S, Chieffi Baccari G. Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia. International Journal of Molecular Sciences. 2016; 17(7):1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071127
Chicago/Turabian StyleDi Fiore, Maria Maddalena, Alessandra Santillo, Sara Falvo, Salvatore Longobardi, and Gabriella Chieffi Baccari. 2016. "Molecular Mechanisms Elicited by d-Aspartate in Leydig Cells and Spermatogonia" International Journal of Molecular Sciences 17, no. 7: 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071127