Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics
Abstract
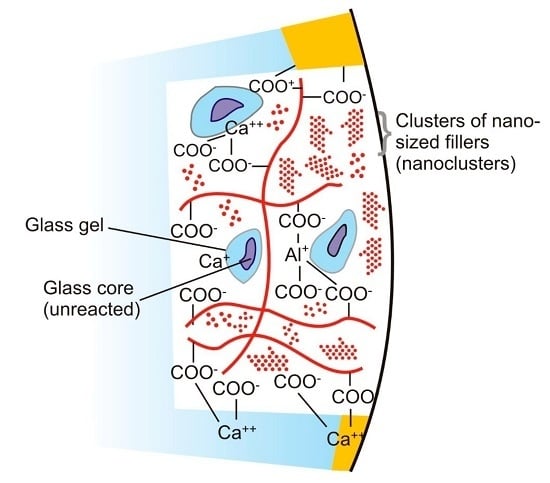
:1. Introduction
2. Powder-Modified Nano Glass Ionomers
2.1. Modification Using Nano-Apatite
2.2. Modification with Nano-Sized HAp/Zr, CaF2 and TiO2 Particles
3. Nano-Filled Resin-Modified Glass Ionomer Cements
3.1. Bonding of Nano-RMGIC with Tooth Structure
3.2. Mechanical and Physical Properties of Nano-RMGICs
3.3. Surface Mechanical Properties of RMGICs
3.4. Fluoride Release from Nano-Ionomers
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Nicholson, J.W. The Chemistry of Medical and Dental Materials; Royal Society of Chemistry: Milton, Cambridge, 2002; Volume 3. [Google Scholar]
- McCabe, J.F.; Walls, A. Applied Dental Materials; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials; Elsevier Health Sciences: St. Louis, MO, USA, 2012. [Google Scholar]
- Sauro, S.; Pashley, D. Stabilize to stabilize dentine-bonded interfaces through reminerlizing operative procedures—State of art. Int. J. Adhes. Adhes. 2016, in press. [Google Scholar] [CrossRef]
- Tyas, M.J.; Burrow, M.F. Adhesive restorative materials: A review. Aust. Dent. J. 2004, 49, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.J.; Higham, S.M.; Agalamanyi, E.A.; Mair, L.H. Fluoride recharge of aesthetic dental materials. J. Oral Rehabil. 1999, 26, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Forsten, L. Resin-modified glass ionomer cements: Fluoride release and uptake. Acta Odontol. Scand. 1995, 53, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Forss, H.; Jokinen, J.; Spets-Happonen, S.; Seppä, L.; Luoma, H. Fluoride and mutans streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991, 25, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.J.; Mair, L.H.; Agalamanyi, E.A.; Higham, S.M. Fluoride release from aesthetic dental materials. J. Oral Rehabil. 1999, 26, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Kent, B.E. The glass-ionomer cement, a new translucent dental filling material. J. Appl. Chem. Biotechnol. 1971, 21, 313. [Google Scholar] [CrossRef]
- McLean, J.W.; Gasser, O. Glass-cermet cements. Quintessence Int. 1985, 16, 333–343. [Google Scholar] [PubMed]
- Wilson, A.D. The chemistry of dental cements. Chem. Soc. Rev. 1978, 7, 265–296. [Google Scholar] [CrossRef]
- Davidson, C.L. Advances in glass-ionomer cements. J. Appl. Oral Sci. 2006, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Nicholson, J.W. Acid-Base Cements: Their Biomedical and Industrial Applications; Cambridge University Press: Cambridge, UK, 2005; Volume 3. [Google Scholar]
- Prosser, H.J.; Powis, D.R.; Wilson, A.D. Glass-ionomer cements of improved flexural strength. J. Dent. Res. 1986, 65, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C. Development of glass-ionomer cement systems. Biomaterials 1998, 19, 467–478. [Google Scholar] [CrossRef]
- Guggenberger, R.; May, R.; Stefan, K.P. New trends in glass-ionomer chemistry. Biomaterials 1998, 19, 479–483. [Google Scholar] [CrossRef]
- Nicholson, J.W. Chemistry of glass-ionomer cements: A review. Biomaterials 1998, 19, 485–494. [Google Scholar] [CrossRef]
- Zainuddin, N.; Karpukhina, N.; Hill, R.G.; Law, R.V. A long-term study on the setting reaction of glass ionomer cements by 27 Al MAS-NMR spectroscopy. Dent. Mater. 2009, 25, 290–295. [Google Scholar]
- De Amorim, R.G.; Leal, S.C.; Frencken, J.E. Survival of atraumatic restorative treatment (ART) sealants and restorations: A meta-analysis. Clin. Oral Investig. 2012, 16, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.W. 3-Year clinical evaluation of a compomer, a resin-modified glass ionomer and a resin composite in class III restorations. Am. J. Dent. 1996, 9, 195–198. [Google Scholar] [PubMed]
- Abdalla, A.I.; Alhadainy, H.A.; Garcia-Godoy, F. Clinical evaluation of glass ionomers and compomers in class V carious lesions. Am. J. Dent. 1997, 10, 18–20. [Google Scholar] [PubMed]
- Leevailoj, C.; Platt, J.A.; Cochran, M.A.; Moore, B.K. In vitro study of fracture incidence and compressive fracture load of all-ceramic crowns cemented with resin-modified glass ionomer and other luting agents. J. Prosthet. Dent. 1998, 80, 699–707. [Google Scholar] [CrossRef]
- Pascotto, R.C.; de Lima Navarro, M.F.; Capelozza Filho, L.; Cury, J.A. In vivo effect of a resin-modified glass ionomer cement on enamel demineralization around orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 36–41. [Google Scholar] [CrossRef]
- Jokstad, A.; Mjör, I.A. Ten years’ clinical evaluation of three luting cements. J. Dent. 1996, 24, 309–315. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; de Munck, J.; Mine, A.; van Meerbeek, B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent. Mater. 2014, 30, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Terata, R.; Nakashima, K.; Kubota, M. Effect of temporary materials on bond strength of resin-modified glass-ionomer luting cements to teeth. Am. J. Dent. 2000, 13, 209–211. [Google Scholar] [PubMed]
- McLean, J.W.; Powis, D.R.; Prosser, H.J.; Wilson, A.D. The use of glass-ionomer cements in bonding composite resins to dentine. Br. Dent. J. 1985, 158, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Wenckert, I.E.; van Dijken, J.W.; Kieri, C. Durability of extensive class II open-sandwich restorations with a resin-modified glass ionomer cement after 6 years. Am. J. Dent. 2004, 17, 43–50. [Google Scholar] [PubMed]
- Frencken, J.E.; Makoni, F.; Sithole, W.D. Art restorations and glass ionomer sealants in Zimbabwe: Survival after 3 years. Community Dent. Oral Epidemiol. 1998, 26, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Yamaga, R.; Nishino, M.; Yoshida, S.; Yokomizo, I. Diammine silver fluoride and its clinical application. J. Osaka Univ. Dent. Sch. 1972, 12, 1–20. [Google Scholar] [PubMed]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin–dentin interfaces created with ion-releasing resin-based systems. Dent. Mater. 2015, 31, 759–777. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Watson, T.F.; Thompson, I.; Toledano, M.; Nucci, C.; Banerjee, A. Influence of air-abrasion executed with polyacrylic acid-bioglass 45S5 on the bonding performance of a resin-modified glass ionomer cement. Eur. J. Oral Sci. 2012, 120, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Jonck, L.M.; Grobbelaar, C.J.; Strating, H. The biocompatibility of glass-ionomer cement in joint replacement: Bulk testing. Clin. Mater. 1989, 4, 85–107. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Wataha, J.C. Dental Materials: Properties and Manipulation; Elsevier Health Sciences: St. Louis, MO, USA, 2014. [Google Scholar]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef]
- Peutzfeldt, A.; Garcia-Godoy, F.; Asmussen, E. Surface hardness and wear of glass ionomers and compomers. Am. J. Dent. 1997, 10, 15–17. [Google Scholar] [PubMed]
- Ahmed, N.; Zafar, M.S. . Effects of wear on hardness and stiffness of restorative dental materials. Life Sci. J. 2014, 11, 11–18. [Google Scholar]
- Um, C.M.; Øilo, G. The effect of early water contact on glass-ionomer cements. Quintessence Int. 1992, 1, 23. [Google Scholar]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I.U. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Resin-modified glass-ionomer cements. Int. J. Prosthodont. 1989, 3, 425–429. [Google Scholar]
- Soncini, J.A.; Maserejian, N.N.; Trachtenberg, F.; Tavares, M.; Hayes, C. The Longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: Findings from the new england children’s amalgam trial. J. Am. Dent. Assoc. 2007, 138, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Khouw-Liu, V.H.W.; Anstice, H.M.; Pearson, G.J. An in vitro investigation of a poly (vinyl phosphonic acid) based cement with four conventional glass-ionomer cements. Part 1: Flexural strength and fluoride release. J. Dent. 1999, 27, 351–357. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of polyelectrolyte modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2012, 22, 2824–2833. [Google Scholar] [CrossRef]
- Lohbauer, U.; Walker, J.; Nikolaenko, S.; Werner, J.; Clare, A.; Petschelt, A.; Greil, P. Reactive fibre reinforced glass ionomer cements. Biomaterials 2003, 24, 2901–2907. [Google Scholar] [CrossRef]
- Gu, Y.W.; Yap, A.U.J.; Cheang, P.; Khor, K.A. Zirconia–glass ionomer cement—A potential substitute for miracle mix. Scr. Mater. 2005, 52, 113–116. [Google Scholar] [CrossRef]
- Gu, Y.W.; Yap, A.U.; Cheang, P.; Khor, K.A. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC). Biomaterials 2005, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Zoergiebel, J.; Ilie, N. Evaluation of a conventional glass ionomer cement with new zinc formulation: Effect of coating, aging and storage agents. Clin. Oral Investig. 2013, 17, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Towler, M.R. The processing, mechanical properties and bioactivity of zinc based glass ionomer cements. J. Mater. Sci. Mater. Med. 2005, 16, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Nicholson, J.W. The effect of strontium oxide in glass–ionomer cements. J. Mater. Sci. Mater. Med. 1999, 10, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Kerby, R.E.; Bleiholder, R.F. Physical properties of stainless-steel and silver-reinforced glass-ionomer cements. J. Dent. Res. 1991, 70, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.; Irie, M.; Yoshida, Y.; Suzuki, K. Effect of adding spherical silica filler on physico-mechanical properties of resin modified glass-ionomer cement. Dent. Mater. J. 2004, 23, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.C.; Culbertson, B.M.; Xie, D. Preparation of glass ionomer cement using N-acryloyl-substituted amino acid monomers—Evaluation of physical properties. Dent. Mater. 1996, 12, 44–51. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified peek dental implants: Bioactive composites and surface modification—A review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnology for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.R.; Palin, W.M.; Fleming, G.J.P.; Shortall, A.C.C.; Marquis, P.M. The mechanical properties of nanofilled resin-based composites: The impact of dry and wet cyclic pre-loading on bi-axial flexure strength. Dent. Mater. 2009, 25, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Terry, D.A. Direct applications of a nanocomposite resin system: Part 1-the evolution of contemporary composite materials. Pract. Proced. Aesthet. Dent. 2004, 16, 417–432. [Google Scholar] [PubMed]
- Chen, M.-H. Update on dental nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef] [PubMed]
- De Caluwe, T.; Vercruysse, C.W.; Fraeyman, S.; Verbeeck, R.M. The influence of particle size and fluorine content of aluminosilicate glass on the glass ionomer cement properties. Dent. Mater. 2014, 30, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Vohra, F.; Zafar, S.; Almas, K. Significance of osteogenic surface coatings on implants to enhance osseointegration under osteoporotic-like conditions. Implant Dent. 2014, 23, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 2000, 28. [Google Scholar] [CrossRef]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zakir, M.; Al Kheraif, A.A.A.; Asif, M.; Wong, F.S.L.; Rehman, I.U. A comparison of the mechanical properties of a modified silorane based dental composite with those of commercially available composite material. Dent. Mater. 2013, 29, e53–e59. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.J.; Pek, Y.S.; Kumar, R.A.; Cheang, P.; Khor, K.A. Experimental studies on a new bioactive material: Haionomer cements. Biomaterials 2002, 23, 955–962. [Google Scholar] [CrossRef]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, Y.K.; Choi, B.J.; Lee, J.H.; Choi, H.J.; Son, H.K.; Hwang, J.W.; Kim, S.O. Physical properties of resin-reinforced glass ionomer cement modified with micro and nano-hydroxyapatite. J. Nanosci. Nanotechnol. 2010, 10, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Kanda, Y.; Nakajima, H.; Sakagami, H. Effects of tio2 nano glass ionomer cements against normal and cancer oral cells. In Vivo 2014, 28, 895–907. [Google Scholar] [PubMed]
- Hall, S.; Bradley, T.; Moore, J.T.; Kuykindall, T.; Minella, L. Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on tio2 toxicity. Nanotoxicology 2009, 3, 91–97. [Google Scholar] [CrossRef]
- McCabe, J.F. Resin-modified glass-ionomers. Biomaterials 1998, 19, 521–527. [Google Scholar] [CrossRef]
- Cattani-Lorente, M.A.; Dupuis, V.; Payan, J.; Moya, F.; Meyer, J.M. Effect of water on the physical properties of resin-modified glass ionomer cements. Dent. Mater. 1999, 15, 71–78. [Google Scholar] [CrossRef]
- Coutinho, E.; Cardoso, M.V.; de Munck, J.; Neves, A.A.; van Landuyt, K.L.; Poitevin, A.; Peumans, M.; Lambrechts, P.; van Meerbeek, B. Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass-ionomer. Dent. Mater. 2009, 25, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, F.; Nassif, M. Bonding nano-filled resin-modified glass ionomer to dentin using different self-etch adhesives. Oper. Dent. 2011, 36, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B. Adhesion to dentin and physical properties of a light-cured glass-ionomer liner/base. J. Dent. Res. 1991, 70, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; McIntyre, N.S.; Davidson, R.D. Studies on the adhesion of glass-ionomer cements to dentin. J. Dent. Res. 1992, 71, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Nakaoki, Y.; Nikaido, T.; Pereira, P.N.R.; Inokoshi, S.; Tagami, J. Dimensional changes of demineralized dentin treated with hema primers. Dent. Mater. 2000, 16, 441–446. [Google Scholar] [CrossRef]
- Kugel, G.; Ferrari, M. The science of bonding: From first to sixth generation. J. Am. Dent. Assoc. 2000, 131, 20S–25S. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Prosser, H.J.; Powis, D.M. Mechanism of adhesion of polyelectrolyte cements to hydroxyapatite. J. Dent. Res. 1983, 62, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Gurgan, S.; Firat, E.; Nathanson, D. Shear bond strength of three different nano-restorative materials to dentin. Oper. Dent. 2010, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Imbery, T.A.; Namboodiri, A.; Duncan, A.; Amos, R.; Best, A.M.; Moon, P.C. Evaluating dentin surface treatments for resin-modified glass ionomer restorative materials. Oper. Dent. 2013, 38, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hamama, H.H.; Burrow, M.F.; Yiu, C. Effect of dentine conditioning on adhesion of resin-modified glass ionomer adhesives. Aust. Dent. J. 2014, 59, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Schmalz, G. The biocompatibility of glass-ionomer cement materials. A status report for the american journal of dentistry. Am. J. Dent. 2001, 14, 387–396. [Google Scholar] [PubMed]
- Hoshika, S.; Munck, J.D.; Sano, H.; Sidhu, S.K.; van Meerbeek, B. Effect of conditioning and aging on the bond strength and interfacial morphology of glass-ionomer cement bonded to dentin. J. Adhes. Dent. 2015, 17, 141–146. [Google Scholar] [PubMed]
- Takahashi, M.; Nakajima, M.; Tagami, J.; Scheffel, D.L.S.; Carvalho, R.M.; Mazzoni, A.; Cadenaro, M.; Tezvergil-Mutluay, A.; Breschi, L.; Tjäderhane, L. The importance of size-exclusion characteristics of type i collagen in bonding to dentin matrices. Acta Biomater. 2013, 9, 9522–9528. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Ozel, E.; Attar, N.; Ozge Bicer, C. Influence of different conditioning methods on the shear bond strength of novel light-curing nano-ionomer restorative to enamel and dentin. Lasers Med. Sci. 2010, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Dutra-Correa, M.; Saraceni, S.H.; Ciaramicoli, M.T.; Kiyan, V.H. Randomized clinical trial of two resin-modified glass ionomer materials: 1-Year results. Oper. Dent. 2012, 37, 591–601. [Google Scholar] [CrossRef] [PubMed]
- El Wakeel, A.M.; Elkassas, D.W.; Yousry, M.M. Bonding of contemporary glass ionomer cements to different tooth substrates; microshear bond strength and scanning electron microscope study. Eur. J. Dent. 2015, 9, 176–182. [Google Scholar] [PubMed]
- Gu, Y.W.; Yap, A.U.; Cheang, P.; Kumar, R. Spheroidization of glass powders for glass ionomer cements. Biomaterials 2004, 25, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Mathis, R.S.; Ferracane, J.L. Properties of a glass-ionomer/resin-composite hybrid material. Dent. Mater. 1989, 5, 355–358. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Hanaoka, K.; Teranaka, T. Fracture toughness of resin-modified glass ionomer restorative materials: Effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent. Mater. 2003, 19, 747–757. [Google Scholar] [CrossRef]
- Pameijer, C.H.; Garcia-Godoy, F.; Morrow, B.R.; Jefferies, S.R. Flexural strength and flexural fatigue properties of resin-modified glass ionomers. J. Clin. Dent. 2015, 26, 23–27. [Google Scholar] [PubMed]
- Hefferren, J.J.; Koehler, H.M. Foods, Nutrition & Dental Health; Pathotox Publishers: Park Forest South, IL, USA, 1981. [Google Scholar]
- Lin, J.; Zhu, J.; Gu, X.; Wen, W.; Li, Q.; Fischer-Brandies, H.; Wang, H.; Mehl, C. Effects of incorporation of nano-fluorapatite or nano-fluorohydroxyapatite on a resin-modified glass ionomer cement. Acta Biomater. 2011, 7, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.R.; Gonçalves, L.d.S.; Lancellotti, A.C.; Consani, S.; Correr-Sobrinho, L.; Sinhoreti, M.A. Nanohybrid resin composites: Nanofiller loaded materials or traditional microhybrid resins? Oper. Dent. 2009, 34, 551–557. [Google Scholar] [CrossRef] [PubMed]
- St-Georges, A.J.; Bolla, M.; Fortin, D.; Muller-Bolla, M.; Thompson, J.Y.; Stamatiades, P.J. Surface finish produced on three resin composites by new polishing systems. Oper. Dent. 2005, 30, 593. [Google Scholar] [PubMed]
- De Paula, A.B.; Fucio, S.B.; Ambrosano, G.M.; Alonso, R.C.; Sardi, J.C.; Puppin-Rontani, R.M. Biodegradation and abrasive wear of nano restorative materials. Oper. Dent. 2011, 36, 670–677. [Google Scholar] [CrossRef] [PubMed]
- De Fúcio, S.B.; de Paula, A.B.; de Carvalho, F.G.; Feitosa, V.P.; Ambrosano, G.M.; Puppin-Rontani, R.M. Biomechanical degradation of the nano-filled resin-modified glass-ionomer surface. Am. J. Dent. 2012, 25, 315–320. [Google Scholar] [PubMed]
- De Paula, A.B.; de Fucio, S.B.; Alonso, R.C.; Ambrosano, G.M.; Puppin-Rontani, R.M. Influence of chemical degradation on the surface properties of nano restorative materials. Oper. Dent. 2014, 39, E109–E117. [Google Scholar] [CrossRef] [PubMed]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D.; Eichmiller, F.C.; Schumacher, G.E. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J. Biomed. Mater. Res. 2000, 53, 381–391. [Google Scholar] [CrossRef]
- Glasspoole, E.A.; Erickson, R.L.; Davidson, C.L. A fluoride-releasing composite for dental applications. Dent. Mater. 2001, 17, 127–133. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Zhang, N.Z.; Shen, C. Effect of CaF2 content on rate of fluoride release from filled resins. J. Dent. Res. 2005, 84, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Zafar, M.S.; Al-Munawwarah, A.-M.; Arabia, S. Oral and dental delivery of fluoride: A review. Fluoride 2015, 48, 195–204. [Google Scholar]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Sakaguchi, R.L. Craig’s Restorative Dental Materials, 13/e; Elsevier: St. Louis, MO, USA, 2006. [Google Scholar]
- Neelakantan, P.; John, S.; Anand, S.; Sureshbabu, N.; Subbarao, C. Fluoride release from a new glass-ionomer cement. Oper. Dent. 2011, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.B.; Gurgel, C.V.; Rios, D.; Magalhães, A.C.; Buzalaf, M.A.R.; Machado, M.A.d.A.M. Fluoride release profile of a nanofilled resin-modified glass ionomer cement. Braz. Dent. J. 2011, 22, 275–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| GIC Formulation | Mechanical Properties | |||||
|---|---|---|---|---|---|---|
| Liquid | Powder | Nano Filler Percentage and Size | Compressive Strength (MPa) | Tensile Strength (MPa) | Flexural Strength (MPa) | Reference (s) |
| Polyacrylic acid copolymer | Unmodified FAS glass | No nano fillers, glass size: 3.34–9.6 µm | 161 | 11.8 | 14.8 | [45] |
| Polyacrylic acid copolymer | FAS Glass + HA | 5 wt. %, 100–200nm | 178 | 19 | 31 | [44] |
| Polyacrylic acid copolymer | FAS Glass + FA | 5 wt. %, 100–200 | 179 | 23 | 33 | [44] |
| Polymer of AA, NVP, IA (8:1:1) | FAS Glass + HA | 5 wt. %, 100–200 nm | 183.8 | 23.5 | 36 | [45] |
| Polyacrylic acid copolymer | FAS Glass + TiO2 | 3%, size variable | 176.27 | ‒ | 23.17 | [69,70] |
| Polyacrylic acid copolymer | FAS Glass + HA/ZrO2 | 4 vol. %, particle dimension: 20 × 200 nm | 176.30 | 12.67 | ‒ | [52] |
© 2016 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071134
Najeeb S, Khurshid Z, Zafar MS, Khan AS, Zohaib S, Martí JMN, Sauro S, Matinlinna JP, Rehman IU. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. International Journal of Molecular Sciences. 2016; 17(7):1134. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071134
Chicago/Turabian StyleNajeeb, Shariq, Zohaib Khurshid, Muhammad Sohail Zafar, Abdul Samad Khan, Sana Zohaib, Juan Manuel Nuñez Martí, Salvatore Sauro, Jukka Pekka Matinlinna, and Ihtesham Ur Rehman. 2016. "Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics" International Journal of Molecular Sciences 17, no. 7: 1134. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071134