Progress in AQP Research and New Developments in Therapeutic Approaches to Ischemic and Hemorrhagic Stroke
Abstract
:1. Introduction
2. Aquaporins
2.1. Aquaporin 1
2.2. Aquaporin 9
2.3. Aquaporin 4
2.4. Aquaporins 3, 5, 8, and 11
3. Cerebral Edema
3.1. Aquaporins in Vasogenic Edema
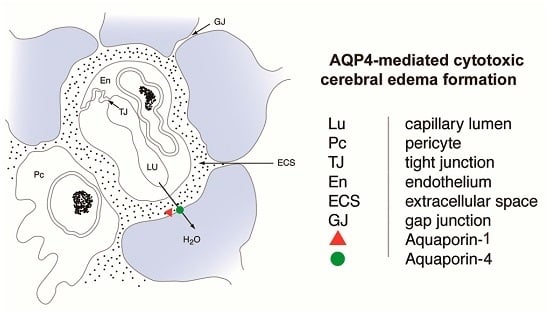
3.2. Aquaporins in Cytotoxic Edema
4. Aquaporin Drug Therapies
4.1. Hormones
4.1.1. Thyroid Hormone
4.1.2. Melatonin
4.2. Loop Diuretics
4.3. Miscellaneous Drugs
4.4. Preconditioning
4.4.1. Chemical Preconditioning
4.4.2. Hyperbaric Oxygen Preconditioning
4.4.3. Remote Limb Ischemic Preconditioning
4.5. Neurotransmitters
4.6. Metabolism-Attenuating Therapies
4.6.1. Ethanol
4.6.2. Therapeutic Hypothermia
5. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | aquaporin |
| BBB | blood brain barrier |
| ICH | intracerebral hemorrhage |
| MCAO | middle cerebral artery occlusion |
| HBO-PC | hyperbaric oxygen preconditioning |
| TH | therapeutic hypothermia |
| RIPC | remote limb ischemic preconditioning |
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Kurland, D.B.; Gerzanich, V.; Simard, J.M. Mechanisms of astrocyte-mediated cerebral edema. Neurochem. Res. 2015, 40, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Arciénega, I.I.; Brunet, J.F.; Bloch, J.; Badaut, J. Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience 2010, 167, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Zammit, C.; Di Giovanni, G.; Muscat, R.; Valentino, M. The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell. Neurosci. 2015, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Buffoli, B.; Barbara, B. Aquaporin biology and nervous system. Curr. Neuropharmacol. 2010, 8, 97–104. [Google Scholar] [PubMed]
- Badaut, J.; Ashwal, S.; Obenaus, A. Aquaporins in cerebrovascular disease: A target for treatment of brain edema? Cerebrovasc. Dis. 2011, 31, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Xue, R.; Haug, F.M.; Neely, J.D.; Bhardwaj, A.; Agre, P.; Adams, M.E.; Froehner, S.C.; Mori, S.; Ottersen, O.P. α-Syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004, 18, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Nico, B.; Ribatti, D.; Svelto, M. Tissue distribution and membrane localization of aquaporin-9 water channel: Evidence for sex-linked differences in liver. J. Histochem. Cytochem. 2001, 49, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Lindland, H.; Zelenin, S.; Roberg, B.A.; Gundersen, B.B.; Petersen, P.; Rinvik, E.; Torgner, I.A.; Ottersen, O.P. Brain mitochondria contain aquaporin water channels: Evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005, 19, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Regli, L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 2004, 129, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Yoneda, K.; Asai, K.; Sobue, K.; Tada, T.; Fujita, Y.; Katsuya, H.; Fujita, M.; Aihara, N.; Mase, M.; et al. Alterations in the expression of the AQP family in cultured rat astrocytes during hypoxia and reoxygenation. Mol. Brain Res. 2001, 90, 26–38. [Google Scholar] [CrossRef]
- Chai, R.C.; Jiang, J.H.; Wong, A.Y.; Jiang, F.; Gao, K.; Vatcher, G.; Hoi Yu, A.C. AQP5 is differentially regulated in astrocytes during metabolic and traumatic injuries. Glia 2013, 61, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Tanaka, Y.; Matsuzaki, T.; Morishita, Y.; Ishibashi, K. Aquaporin-11 (AQP11) Expression in the Mouse Brain. Int. J. Mol. Sci. 2016, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Tait, M.J.; Saadoun, S.; Bell, B.A.; Verkman, A.S.; Papadopoulos, M.C. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience 2010, 167, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C. Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010, 168, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.F.; Cui, Z.W.; Zhong, Z.H.; Sun, Y.H.; Sun, Q.F.; Yang, G.Y.; Bian, L.G. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol. Sin. 2015, 36, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Brunet, J.F.; Grollimund, L.; Hamou, M.F.; Magistretti, P.J.; Villemure, J.G.; Regli, L. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir. Suppl. 2003, 86, 495–498. [Google Scholar] [PubMed]
- De Castro Ribeiro, M.; Hirt, L.; Bogousslavsky, J.; Regli, L.; Badaut, J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J. Neurosci. Res. 2006, 83, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Frydenlund, D.S.; Bhardwaj, A.; Otsuka, T.; Mylonakou, M.N.; Yasumura, T.; Davidson, K.G.; Zeynalov, E.; Skare, O.; Laake, P.; Haug, F.M.; et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 13532–13536. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Pandey, A.K.; Paul, S.; Patnaik, R. Melatonin renders neuroprotection by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci. 2014, 100, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, Y.W.; Park, K.A.; Lee, W.T.; Lee, J.E. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Unabia, G.C.; Johnson, K.; Ye, Z.; Vergara, L.; Hulsebosch, C.E.; Perez-Polo, J.R. Aquaporin 1—A novel player in spinal cord injury. J. Neurochem. 2008, 105, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorgan. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, Y.; Hiroaki, Y.; Fujiyoshi, Y. Acetazolamide reversibly inhibits water conduction by Aquaporin-4. J. Struct. Biol. 2009, 166, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nakayama, S.; Amiry-Moghaddam, M.; Ottersen, O.P.; Bhardwaj, A. Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit. Care 2010, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Taya, K.; Marmarou, C.R.; Ozisik, P.; Fazzina, G.; Kleindienst, A.; Gulsen, S.; Marmarou, A. The modulation of aquaporin-4 by using PKC-activator (phorbol myristate acetate) and V1a receptor antagonist (SR49059) following middle cerebral artery occlusion/reperfusion in the rat. Acta Neurochir. Suppl. 2008, 102, 431–436. [Google Scholar] [PubMed]
- Gunnarson, E.; Song, Y.; Kowalewski, J.M.; Brismar, H.; Brines, M.; Cerami, A.; Andersson, U.; Zelenina, M.; Aperia, A. Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc. Natl. Acad. Sci. USA 2009, 106, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Choi, J.H.; Choi, Y.H.; Park, E.M. Conserved aquaporin 4 levels associated with reduction of brain edema are mediated by estrogen in the ischemic brain after experimental stroke. Biochim. Biophys. Acta 2011, 1812, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Mei, Y.; Guo, Y. Neuregulin attenuated cerebral ischemia—Creperfusion injury via inhibiting apoptosis and upregulating aquaporin-4. Neurosci. Lett. 2008, 443, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Habib, P.; Dang, J.; Slowik, A.; Victor, M.; Beyer, C. Hypoxia-induced gene expression of aquaporin-4, cyclooxygenase-2 and hypoxia-inducible factor 1α in rat cortical astroglia is inhibited by 17β-estradiol and progesterone. Neuroendocrinology 2014, 99, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Sadana, P.; Coughlin, L.; Burke, J.; Woods, R.; Mdzinarishvili, A. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J. Neurol. Sci. 2015, 354, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.R.; Amiry-Moghaddam, M.; Froehner, S.C.; Adams, M.E.; Ottersen, O.P.; Bhardwaj, A. Na+–K+–2Cl− cotransport inhibitor attenuates cerebral edema following experimental stroke via the perivascular pool of aquaporin-4. Neurocrit. Care 2010, 13, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chio, C.C.; Chang, C.H.; Kuo, J.R.; Chang, C.P. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pardue, S.; Arnold, T.C.; Monroe, J.; Alexander, J.S.; Carden, D.L.; Turnage, R.; Conrad, S.A. Ifenprodil treatment is associated with a downregulation of brain aquaporin 4 following cardiac arrest in rats. Acta Neurochir. Suppl. 2005, 95, 415–419. [Google Scholar] [PubMed]
- Li, M.; Ma, R.N.; Li, L.H.; Qu, Y.Z.; Gao, G.D. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur. J. Pharmacol. 2013, 715, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, B.; Dai, M.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci. Lett. 2013, 555, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.T.; Zhang, H.; Xue, Y.X. Dexamethasone treatment modulates aquaporin-4 expression after intracerebral hemorrhage in rats. Neurosci. Lett. 2007, 413, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kawahara, K.; Tancharoen, S.; Matsuda, F.; Morimoto, Y.; Ito, T.; Biswas, K.K.; Takenouchi, K.; Miura, N.; Oyama, Y.; et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J. Pharmacol. Exp. Ther. 2009, 329, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tancharoen, S.; Matsuda, F.; Biswas, K.K.; Ito, T.; Morimoto, Y.; Oyama, Y.; Takenouchi, K.; Miura, N.; Arimura, N.; et al. Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochem. Biophys. Res. Commun. 2009, 390, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Asmaro, K.; Ren, C.; Gao, M.; Peng, C.; Ding, J.Y.; Fredrickson, V.; Ji, X.; Ding, Y. Acute ethanol treatment reduces blood-brain barrier dysfunction following ischemia/reperfusion injury. Brain Res. 2012, 1437, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, W.A.; Fu, P.; Chakraborty, T.; Hussain, M.; Guthikonda, M.; Rafols, J.A.; Ding, Y. At low doses ethanol maintains blood-brain barrier (BBB) integrity after hypoxia and reoxygenation: A brain slice study. Neurol. Res. 2013, 35, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, H.Q.; Lu, L.; Fu, D.L.; Liu, A.J.; Li, J.H.; Zheng, G.Q. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine 2014, 21, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Sun, W.; Gong, W.; Ding, Y.; Zhuang, Y.; Hou, Q. Ginsenoside Rg1 protects against transient focal cerebral ischemic injury and suppresses its systemic metabolic changes in cerabral injury rats. Acta Pharm. Sin. B 2015, 5, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, B.; Cheng, L.; Chi, M.; Deng, L.; Pan, H.; Yao, X.; Wang, G. Hydrogen sulfide induces neuroprotection against experimental stroke in rats by downregulation of AQP4 via activating PKC. Brain Res. 2015, 1622, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Fazzina, G.; Amorini, A.M.; Marmarou, C.R.; Fukui, S.; Okuno, K.; Dunbar, J.G.; Glisson, R.; Marmarou, A.; Kleindienst, A. The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J. Neurotrauma 2010, 27, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Pandey, A.K.; Paul, S.; Patnaik, R.; Yavagal, D.R. Aquaporin-4 inhibition mediates piroxicam-induced neuroprotection against focal cerebral ischemia/reperfusion injury in rodents. PLoS ONE 2013, 8, e73481. [Google Scholar]
- Xiong, X.X.; Gu, L.J.; Shen, J.; Kang, X.H.; Zheng, Y.Y.; Yue, S.B.; Zhu, S.M. Probenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in mice. Neurochem. Res. 2014, 39, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.T.; Liang, J.J.; Miao, L.P.; Wu, Q.; Cao, M.H. Propofol post-conditioning protects the blood brain barrier by decreasing matrix metalloproteinase-9 and aquaporin-4 expression and improves the neurobehavioral outcome in a rat model of focal cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2015, 12, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cui, H.S.; Shin, S.K.; Kim, J.M.; Kim, S.Y.; Lee, J.E.; Koo, B.N. Effect of propofol post-treatment on blood-brain barrier integrity and cerebral edema after transient cerebral ischemia in rats. Neurochem. Res. 2013, 38, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Lu, C.; Xia, C.M.; Qiao, Z.W.; Zhu, D.N. Simvastatin pretreatment protects cerebrum from neuronal injury by decreasing the expressions of phosphor-CaMK II and AQP4 in ischemic stroke rats. J. Mol. Neurosci. 2014, 54, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Tsujita, M.; Suzuki, Y.; Kwee, I.L.; Nakada, T. Inhibition of aquaporin-4 significantly increases regional cerebral blood flow. Neuroreport 2013, 24, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yamamoto, T.; Shimizu, K.; Sugiura, Y.; Ugawa, Y. Chemical preconditioning-induced reactive astrocytosis contributes to the reduction of post-ischemic edema through aquaporin-4 downregulation. Exp. Neurol. 2011, 227, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Tsunoda, A.; Yamamoto, T.; Tada, M.; Kakita, A.; Ugawa, Y. Increased neuronal and astroglial aquaporin-1 immunoreactivity in rat striatum by chemical preconditioning with 3-nitropropionic acid. Neurosci. Lett. 2016, 626, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hirt, L.; Ternon, B.; Price, M.; Mastour, N.; Brunet, J.F.; Badaut, J. Protective role of early aquaporin 4 induction against postischemic edema formation. J. Cereb. Blood Flow Metab. 2009, 29, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Abumiya, T.; Nakamura, H.; Shimbo, D.; Shichinohe, H.; Nakayama, N.; Kazumata, K.; Shimizu, H.; Houkin, K. Transarterial regional brain hypothermia inhibits acute aquaporin-4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery 2016, 79, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, C.S.; Gong, P.; Tang, Z.R.; Hua, R.; Mei, X.; Zhang, M.Y.; Cui, J. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation 2012, 83, 913–920. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, X.; Wu, Y.; Jia, J.; Hu, Y.; Yang, X.; Li, J.; Fan, M.; Zhang, L.; Guo, J.; et al. Treadmill pre-training ameliorates brain edema in ischemic stroke via downregulation of aquaporin-4: An MRI study in rats. PLoS ONE 2014, 9, e84602. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, H.; Li, G.; Wang, L. Effect of hyperbaric oxygen preconditioning on peri-hemorrhagic focal edema and aquaporin-4 expression. Exp. Ther. Med. 2015, 10, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Sun, M.; He, P.P.; Wen, L.L.; Zhang, H.; Feng, J. Ischemic postconditioning alleviates brain edema after focal cerebral ischemia reperfusion in rats through down-regulation of aquaporin-4. J. Mol. Neurosci. 2015, 56, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Sun, H.; Chen, S.; Wang, F. Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS ONE 2014, 9, e97488. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Y.; Zhang, Z.; Lu, Y.; Wang, Y.; Huang, J.; Li, Y.; Chen, X.; Gu, X.; Wang, Y.; et al. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells 2014, 32, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Sepramaniam, S.; Ying, L.K.; Armugam, A.; Wintour, E.M.; Jeyaseelan, K. MicroRNA-130a represses transcriptional activity of aquaporin 4 M1 promoter. J. Biol. Chem. 2012, 287, 12006–12015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.B. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J. Cereb. Blood Flow Metab. 2015, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Sepramaniam, S.; Armugam, A.; Lim, K.Y.; Karolina, D.S.; Swaminathan, P.; Tan, J.R.; Jeyaseelan, K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J. Biol. Chem. 2010, 285, 29223–29230. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, P.; Su, J.; Xiang, J.; Cai, D.; Dong, Q. Effects of aquaporin-4 on edema formation following intracerebral hemorrhage. Exp. Neurol. 2010, 223, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ambrosius, W.; Kazmierski, R.; Gupta, V.; Warot, A.W.; Adamczewska-Kocialkowska, D.; Blazejewska, A.; Ziemnicka, K.; Nowinski, W.L. Low free triiodothyronine levels are related to poor prognosis in acute ischemic stroke. Exp. Clin. Endocrinol. Diabetes 2011, 119, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Yamamoto, M.; Takei, N.; Kumazaki, M.; Ungsuparkorn, C.; Hida, H.; Sanberg, P.R.; Nishino, H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000, 14, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sivakumar, V.; Zhang, Y.; Ling, E.A. Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia 2006, 54, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Töllner, K.; Brandt, C.; Römermann, K.; Löscher, W. The organic anion transport inhibitor probenecid increases brain concentrations of the NKCC1 inhibitor bumetanide. Eur. J. Pharmacol. 2015, 746, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Pandey, A.K.; Paul, S.; Patnaik, R. Neuroprotective potential of Piroxicam in cerebral ischemia: An in silico evaluation of the hypothesis to explore its therapeutic efficacy by inhibition of aquaporin-4 and acid sensing ion channel1a. Med. Hypotheses 2012, 79, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.K.; Borah, A. Piroxicam confer neuroprotection in Cerebral Ischemia by inhibiting cyclooxygenases, acid- sensing ion channel-1a and aquaporin-4: An in silico comparison with Aspirin and Nimesulide. Bioinformation 2015, 11, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Lan, Y.P.; Tang, H.F.; Zhu, S.M. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesth. Analg. 2008, 107, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, J.; Xu, J.; Sheng, G.; Huang, G. Propofol administration modulates AQP-4 expression and brain edema after traumatic brain injury. Cell Biochem. Biophys. 2013, 67, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, S.; Kamiya, T.; Nito, C.; Inaba, T.; Kato, K.; Ueda, M.; Shimazaki, K.; Katayama, Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur. J. Pharmacol. 2005, 516, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Wang, M.M.; Xiang, J.; Hua, Y.; Xi, G. Is there a place for cerebral preconditioning in the clinic? Transl. Stroke Res. 2010, 1, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Sunami, K.; Takeda, Y.; Hashimoto, M.; Hirakawa, M. Hyperbaric oxygen reduces infarct volume in rats by increasing oxygen supply to the ischemic periphery. Crit. Care Med. 2000, 28, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xi, G.; Keep, R.F.; Silbergleit, R.; He, Y.; Hua, Y. Hyperbaric oxygen for experimental intracerebral hemorrhage. Acta Neurochir. Suppl. 2008, 105, 113–117. [Google Scholar] [PubMed]
- Li, S.; Hu, X.; Zhang, M.; Zhou, F.; Lin, N.; Xia, Q.; Zhou, Y.; Qi, W.; Zong, Y.; Yang, H.; et al. Remote ischemic post-conditioning improves neurological function by AQP4 downregulation in astrocytes. Behav. Brain Res. 2015, 289, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, F.; Li, S.; Zong, Y.; Zhang, M.; Lin, Y.; Zhang, X.; Yang, H.; Zou, Y.; Qi, C.; et al. Remote ischemic postconditioning protects ischemic brain from injury in rats with focal cerebral ischemia/reperfusion associated with suppression of TLR4 and NF-κB expression. Neuroreport 2016, 27, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Ren, C.; Wang, T.; Fu, P.; Luo, Y.; Liu, X.; Yan, F.; Ling, F.; Jia, J.; Du, H.; et al. Effect of remote ischemic postconditioning on an intracerebral hemorrhage stroke model in rats. Neurol. Res. 2012, 34, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, Y.; Li, L.; Guan, X.; Wang, D. Effects of local hypothermia on neuronal cell apoptosis after intracerebral hemorrhage in rats. J. Nutr. Health Aging 2015, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, J.; Li, J.; Wang, L.; Hall, C.L.; Dix, T.A.; Mohamad, O.; Wei, L.; Yu, S.P. Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice. Neuroscience 2013, 252, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wei, L.; Gu, X.; Won, S.; Wei, Z.Z.; Dix, T.A.; Yu, S.P. Improved therapeutic benefits by combining physical cooling with pharmacological hypothermia after severe stroke in rats. Stroke 2016, 47, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Beard, D.J.; Bourke, J.T.; Pepperall, D.; McLeod, D.D.; Spratt, N.J. Intracranial pressure elevation 24 h after ischemic stroke in aged rats is prevented by early, short hypothermia treatment. Front. Aging Neurosci. 2016, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ding, F.; Lei, G.; Luan, G.; Zhang, S.; Li, K.; Wang, D.; Zhang, L.; Dai, D. Effects of focal mild hypothermia on thrombin-induced brain edema formation and the expression of protease activated receptor-1, matrix metalloproteinase-9 and aquaporin 4 in rats. Mol. Med. Rep. 2015, 11, 3009–3014. [Google Scholar] [PubMed]

| Therapy | Aquaporin-4 Expression (after Stroke and Treatment) | Model | Time Points (Reperfusion Time) | References | Year |
|---|---|---|---|---|---|
| Drug Therapies | |||||
| Arylsulfonamides | |||||
| 4-acetamido-benzsulfonamide | Decreased permeability | Xenopusoocytes | N/A | Huber et al. [25] | 2007 |
| Acetazolamide (AZA) | Decreased permeability | Xenopusoocytes | N/A | Huber et al. [25] | 2007 |
| Decreased permeability | Aquaporin 4 proteins within liposomes | N/A | Tanimura et al. [26] | 2009 | |
| 6-Ethoxy-benzothiazole-2-sulfonamide (EZA) | Decreased permeability | Xenopusoocytes | N/A | Huber et al. [25] | 2007 |
| Hormones and hormone receptor modulators | |||||
| Arginine vasopressin (AVP) V1 receptor antagonist (SR 49059) | Decreased | Mouse transient middle cerebral artery occlusion (MCAO), 60 min | 24 h | Liu et al. [27] | 2010 |
| Decreased | Rat transient MCAO, 2 h | 2 h | Okuno et al. [28] | 2008 | |
| Erythropoietin | Decreased | Mouse primary brain edema | N/A | Gunnarson et al. [29] | 2009 |
| Estrogen | Decreased | Mouse transient MCAO, 30 min | 3 days | Shin et al. [30] | 2011 |
| Melatonin | Decreased | Rat transient MCAO, 1 h | 24 h | Bhattacharya et al. [22] | 2014 |
| Neuregulin-1β | Decreased | Rat transient MCAO, 90 min | 0.5, 1, 1.5, and 2 h | Li et al. [31] | 2008 |
| Progesterone | Decreased | Rat astroglial cell culture, 3 h hypoxia | N/A | Habib et al. [32] | 2014 |
| Triiodothyronine | Decreased | Mouse transient MCAO, 60 min | 24 h | Sadana et al. [33] | 2015 |
| Na+/K+/Cl− channel blockers (loop diuretics) | |||||
| AqB013 (4-aminopyridine carboxamide analog) | Decreased aquaporin-4 permeability | Xenopus laevis oocyte | N/A | Migliati et al. [7] | 2009 |
| Bumetanide | Decreased permeability | Xenopus oocytes | N/A | Migliati et al. [7] | 2009 |
| Decreased | Rat transient MCAO, 90 min | 24 h, and 2 days | Migliati et al. [34] | 2010 | |
| Furosemide | Decreased aquaporin-4 permeability | Xenopus laevis oocyte | N/A | Migliati et al. [7] | 2009 |
| Neurotransmitters and neurotransmitter modulators | |||||
| Agmatine | Decreased aquaporin-4 positive cells | Rat transient MCAO, 90 min | 4 days | Wang et al. [35] | 2010 |
| Ifenprodil | Decreased | Rat cardiac arrest model | N/A | Xiao et al. [36] | 2005 |
| Other organic molecules | |||||
| Astragaloside IV | Decreased | Rat transient MCAO, 90 min | 24 h | Li et al. [37] | 2013 |
| Carvacrol | Decreased mRNA and protein levels (perihematomal area) | Rat intracerebral hemorrhage | 3 days | Zhong et al. [38] | 2013 |
| Dexamethasone | Decreased mRNA levels (perihematomal area) | Rat intracerebral hemorrhage | 24 h | Gu et al. [39] | 2007 |
| Edaravone | Decreased | Rat transient MCAO, 90 min | 24 h | Kikuchi et al. [40,41] | 2009 |
| Ethanol | Decreased | Rat transient MCAO, 2 h | 3 and 24 h | Zeng et al. [42,43] | 2012 |
| Ginsenoside Rg1 | Decreased | Rat transient MCAO, 2 h | 6, 24 h, 3, 7, and 14 days | Zhou et al. [44,45] | 2014 |
| Hydrogen sulfide | Decreased | Rat transient MCAO, 2 h | 24 h | Wei et al. [46] | 2015 |
| Phorbol myristate acetate (PMA) | Decreased | Rat transient MCAO, 2 h | 2 h | Okuno et al. [28,47] | 2008 |
| Piroxicam | Decreased | Rat transient MCAO, 1 h | 24 h | Bhattacharya et al. [48] | 2013 |
| Probenecid | Decreased | Mouse transient MCAO, 1 h | 48 h | Xiong et al. [49] | 2014 |
| Propofol | Decreased | Rat transient MCAO, 2 h | 24 h | Ji et al. [50] | 2015 |
| Decreased | Rat transient MCAO, 1 h | 24 h | Lee et al. [51] | 2013 | |
| Simvastatin | Decreased | Rat transient MCAO, 1 h | 24 h | Zhu et al. [52] | 2014 |
| TGN-020 | Decreased | Mouse transient MCAO, 2 h | 24 h | Igarashi et al. [53,54] | 2011 |
| Pharmacological preconditioning | |||||
| 3-Nitroproprionic acid | Decreased | Rat transient MCAO, 2 h | 4 days | Hoshi et al. [55,56] | 2011 |
| Thrombin | Increased | Mouse transient MCAO, 30 min | 1, 24, and 2 day | Hirt et al. [57] | 2009 |
| Non-Drug Therapies | |||||
| Therapeutic hypothermia | |||||
| Internal carotid endovascular infusion | Decreased | Rat transient MCAO, 2 h | 0, 2, 6, 24 h | Kurisu et al. [58] | 2015 |
| External jugular endovascular infusion | Decreased | Pig cardiopulmonary resuscitation | 24 h was significant | Zhao et al. [59] | 2012 |
| Stress preconditioning | |||||
| Exercise pre-training | Decreased | Rat transient MCAO, 90 min | 1, 2.5, 7.5 h, 1, 2, 3 days | He et al. [60] | 2014 |
| Hyperbaric oxygen preconditioning | Decreased | Rat intracerebral hemorrhage | 24 h, 2, 3, 5, and 7 days | Fang et al. [61] | 2015 |
| Remote limb ischemic preconditioning | Decreased | Rat transient MCAO, 2 h | 24 h | Han et al. [62] | 2015 |
| Other Therapies | |||||
| GV20 and ST36 acupuncture | Decreased | Rat transient MCAO, 2 h | 24 h | Xu et al. [63] | 2014 |
| Mesenchymal stem cells | Decreased | Mouse transient MCAO, 90 min | 24 h, and 3 days | Tang et al. [64] | 2014 |
| RNA targets | |||||
| miRNA-130a | Decreased | Rat transient MCAO, 60 min | 24 h | Sepramaniam et al. [65] | 2012 |
| Decreased | Human astrocytoma cell culture | N/A | |||
| miRNA-29b | Increased | Mouse transient MCAO, 3 h | 24 h, and 3 days | Wang et al. [66] | 2015 |
| miRNA-320a | Decreased | Rat transient MCAO, 60 min | 24 h | Sepramaniam et al. [67] | 2010 |
| Decreased | Human astrocytoma cell culture | N/A | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Previch, L.E.; Ma, L.; Wright, J.C.; Singh, S.; Geng, X.; Ding, Y. Progress in AQP Research and New Developments in Therapeutic Approaches to Ischemic and Hemorrhagic Stroke. Int. J. Mol. Sci. 2016, 17, 1146. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071146
Previch LE, Ma L, Wright JC, Singh S, Geng X, Ding Y. Progress in AQP Research and New Developments in Therapeutic Approaches to Ischemic and Hemorrhagic Stroke. International Journal of Molecular Sciences. 2016; 17(7):1146. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071146
Chicago/Turabian StylePrevich, Lauren E., Linlin Ma, Joshua C. Wright, Sunpreet Singh, Xiaokun Geng, and Yuchuan Ding. 2016. "Progress in AQP Research and New Developments in Therapeutic Approaches to Ischemic and Hemorrhagic Stroke" International Journal of Molecular Sciences 17, no. 7: 1146. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071146