The Conserved Arginine Cluster in the Insert of the Third Cytoplasmic Loop of the Long Form of the D2 Dopamine Receptor (D2L-R) Acts as an Intracellular Retention Signal
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Generated Long Form of the D2 Dopamine Receptor (D2L-R) Mutants
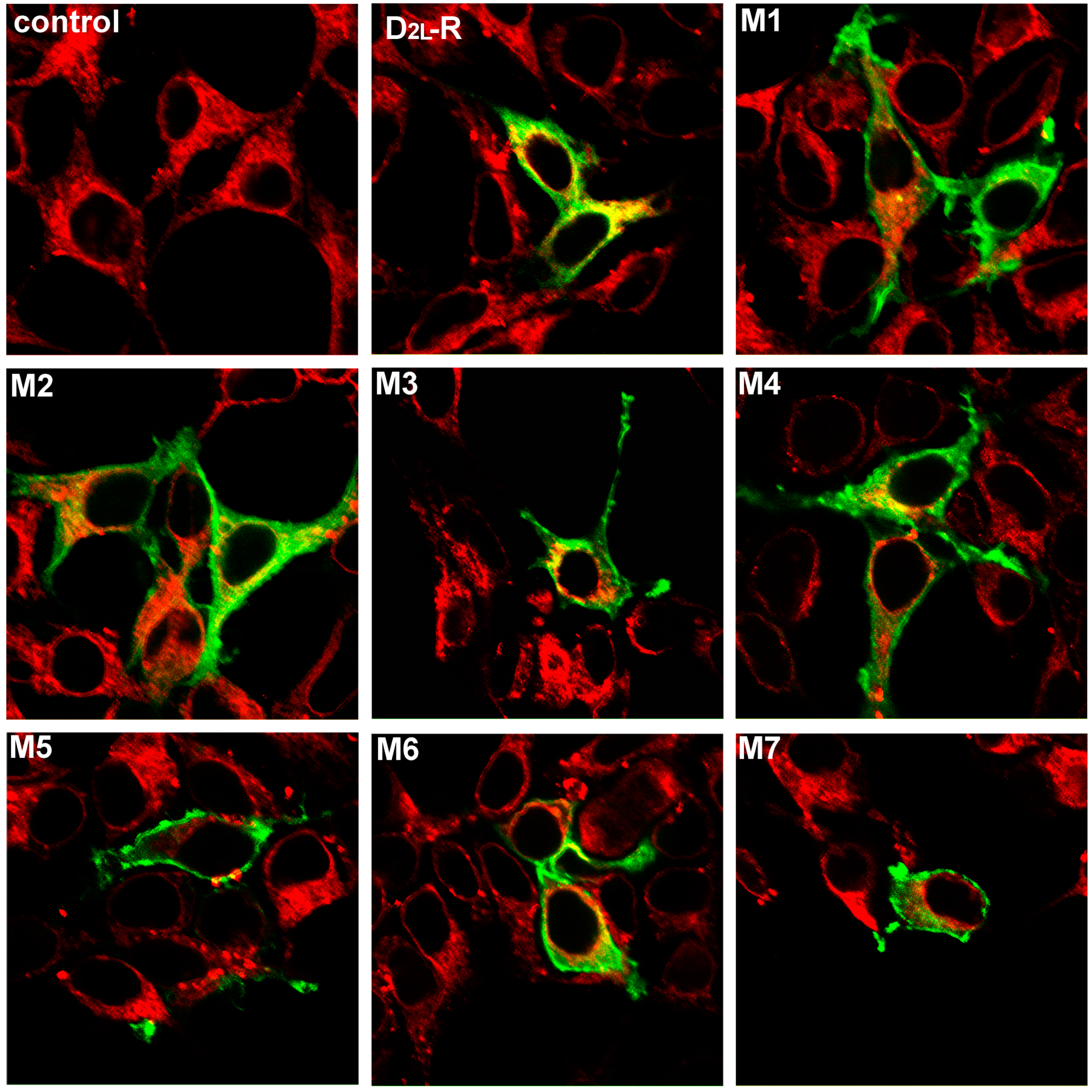
2.2. Visualization and Cellular Localization of the D2L-Rs—Colocalization Study
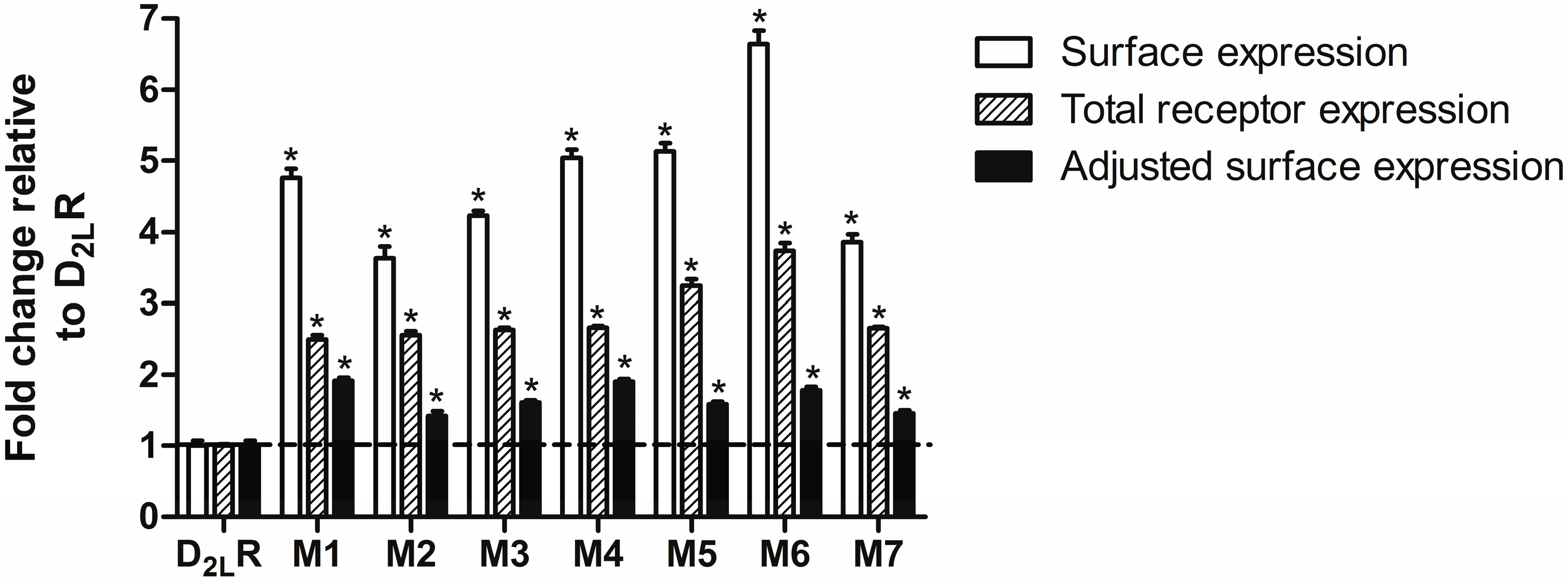
2.3. D2L-R Constructs Surface Expression—Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. D2L-R Constructs Functional Characterization—Bioluminescence Resonance Energy Transfer (BRET2) and cAMP Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Receptor and β-Arrestin 2 Constructs
4.3. Cell Culture and Transfection
4.4. Confocal Microscopyand Quantification of Co-Localization
4.5. Radiolig and Binding Assay
4.6. Luminescence and Fluorescence Measurements
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. BRET-Based β-Arrestin 2 Recruitments Assay
4.9. cAMP Assay
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Eidne, K.A.; Taylor, P.L.; Zabavnik, J.; Saunders, P.T.; Inglis, J.D. D2 receptor, a missing exon. Nature 1989, 342, 865. [Google Scholar] [CrossRef] [PubMed]
- Giros, B.; Sokoloff, P.; Martres, M.P.; Riou, J.F.; Emorine, L.J.; Schwartz, J.C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 1989, 342, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Monsma, F.J.; Mcvittie, L.D.; Gerfen, C.R.; Mahan, L.C.; Sibley, D.R. Multiple D2 dopamine-receptors produced by alternative rna splicing. Nature 1989, 342, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Reilander, H.; Michel, H. In vivo reconstitution of dopamine D2S receptor-mediated G protein activation in baculovirus-infected insect cells: Preferred coupling to Gi1 versus Gi2. Biochemistry 1996, 35, 15162–15173. [Google Scholar] [CrossRef] [PubMed]
- Montmayeur, J.P.; Guiramand, J.; Borrelli, E. Preferential coupling between dopamine-D2 receptors and G-proteins. Mol. Endocrinol. 1993, 7, 161–170. [Google Scholar] [PubMed]
- Senogles, S.E. The D2 dopamine receptor isoforms signal through distinct Giα proteins to inhibit adenylyl cyclase. A study with site-directed mutant Giα proteins. J. Biol. Chem. 1994, 269, 23120–23127. [Google Scholar] [PubMed]
- Fishburn, C.S.; Elazar, Z.; Fuchs, S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J. Biol. Chem. 1995, 270, 29819–29824. [Google Scholar] [PubMed]
- Lindgren, N.; Usiello, A.; Goiny, M.; Haycock, J.; Erbs, E.; Greengard, P.; Hokfelt, T.; Borrelli, E.; Fisone, G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc. Natl. Acad. Sci. USA 2003, 100, 4305–4309. [Google Scholar] [CrossRef] [PubMed]
- Usiello, A.; Baik, J.H.; Rougé-Pont, F.; Picetti, R.; Dierich, A.; LeMeur, M.; Piazza, P.V.; Borrelli, E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000, 408, 199–203. [Google Scholar] [PubMed]
- Wang, Y.; Xu, R.; Sasaoka, T.; Tonegawa, S.; Kung, M.P.; Sankoorikal, E.B. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J. Neurosci. 2000, 20, 8305–8314. [Google Scholar] [PubMed]
- Welter, M.; Vallone, D.; Samad, T.A.; Meziane, H.; Usiello, A.; Borrelli, E. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc. Natl. Acad. Sci. USA 2007, 104, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hranilovic, D.; Fetsko, L.A.; Bucan, M.; Wang, Y. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol. Psychiatry 2002, 7, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Mrzljak, L.; Gutierrez, A.; de la Calle, A.; Goldman-Rakic, P.S. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 7731–7736. [Google Scholar] [CrossRef] [PubMed]
- Prou, D.; Gu, W.J.; Le Crom, S.; Vincent, J.D.; Salamero, J.; Vernier, P. Intracellular retention of the two isoforms of the D2 dopamine receptor promotes endoplasmic reticulum disruption. J. Cell Sci. 2001, 114, 3517–3527. [Google Scholar] [PubMed]
- Sedaghat, K.; Nantel, M.F.; Ginsberg, S.; Lalonde, V.; Tiberi, M. Molecular characterization of dopamine D2 receptor isoforms tagged with green fluorescent protein. Mol. Biotechnol. 2006, 34, 1–14. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fukunaga, K. Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells. J. Neurochem. 2003, 85, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.Y.; Varghese, G.; Chung, H.T.; Trogadis, J.; Seeman, P.; O’Dowd, B.F.; George, S.R. Resistance of the dopamine D2L receptor to desensitization accompanies the up-regulation of receptors on to the surface of Sf9 cells. Endocrinology 1997, 138, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Van Craenenbroeck, K.; Clark, S.D.; Cox, M.J.; Oak, J.N.; Liu, F.; van Tol, H.H. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J. Biol. Chem. 2005, 280, 19350–19357. [Google Scholar] [CrossRef] [PubMed]
- Achour, L.; Labbe-Jullie, C.; Scott, M.G.H.; Marullo, S. An escort for GPCRs: Implications for regulation of receptor density at the cell surface. Trends Pharmacol. Sci. 2008, 29, 528–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, H.; Gata, G.; Marullo, S. Regulated GPCR trafficking to the plasma membrane: General issues and theCCR5 chemokine receptor example. Subcell. Biochem. 2012, 63, 97–111. [Google Scholar] [PubMed]
- Dong, C.M.; Filipeanu, C.M.; Duvernay, M.T.; Wu, G.Y. Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 2007, 1768, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G. G protein-coupled receptor hetero-dimerization: Contribution to pharmacology and function. Br. J. Pharmacol. 2009, 158, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Babcock, J.J.; Li, M. Inside job: Ligand-receptor pharmacology beneath the plasma membrane. Acta Pharmacol. Sin. 2013, 34, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Doly, S.; Marullo, S. Gatekeepers controlling GPCR export and function. Trends Pharmacol. Sci. 2015, 36, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Margeta-Mitrovic, M.; Jan, Y.N.; Jan, L.Y. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Restituito, S.; Couve, A.; Bawagan, H.; Jourdain, S.; Pangalos, M.N.; Calver, A.R.; Freeman, K.B.; Moss, S.J. Multiple motifs regulate the trafficking of GABAB receptors at distinct checkpoints within the secretory pathway. Mol. Cell. Neurosci. 2005, 28, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Doly, S.; Shirvani, H.; Gata, G.; Meye, F.J.; Emerit, M.B.; Enslen, H.; Achour, L.; Pardo-Lopez, L.; Yang, S.K.; Armand, V.; et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol. Psychiatry 2016, 4, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerangue, N.; Schwappach, B.; Jan, Y.N.; Jan, L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 1999, 22, 537–548. [Google Scholar] [CrossRef]
- Ma, D.; Zerangue, N.; Lin, Y.F.; Collins, A.; Yu, M.; Jan, Y.N.; Jan, L.Y. Role of ER export signals in controlling surface potassium channel numbers. Science 2001, 291, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Shikano, S. Phosphorylation-dependent C-terminal binding of 14-3-3 proteins promotes cell surface expression of HIV co-receptor GPR15. J. Biol. Chem. 2011, 286, 7171–7181. [Google Scholar] [CrossRef] [PubMed]
- Nasu-Nishimura, Y.; Hurtado, D.; Braud, S.; Tang, T.T.; Isaac, J.T.; Roche, K.W. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J. Neurosci. 2006, 26, 7014–7021. [Google Scholar] [CrossRef] [PubMed]
- Zahniser, N.R.; Dubocovich, M.L. Comparison of dopamine receptor sites labeled by [3H]-S-sulpiride and [3H]-spiperone in striatum. J. Pharmacol. Exp. Ther. 1983, 227, 592–599. [Google Scholar] [PubMed]
- Audinot, V.; Newman-Tancredi, A.; Gobert, A.; Rivet, J.M.; Brocco, M.; Lejeune, F.; Gluck, L.; Desposte, I.; Bervoets, K.; Dekeyne, A.; et al. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. J. Pharmacol. Exp. Ther. 1998, 287, 187–197. [Google Scholar] [PubMed]
- Vrecl, M.; Jorgensen, R.; Pogacnik, A.; Heding, A.; Vrecl, M.; Jorgensen, R.; Pogacnik, A.; Heding, A. Development of a BRET2 screening assay using β-arrestin 2 mutants. J. Biomol. Screen. 2004, 9, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Vrecl, M.; Norregaard, P.K.; Almholt, D.L.C.; Elster, L.; Pogacnik, A.; Heding, A. β-arrestin-based BRET2 screening assay for the “non”-β-arrestin binding CB1 receptor. J. Biomol. Screen. 2009, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Klewe, I.V.; Nielsen, S.M.; Tarpo, L.; Urizar, E.; Dipace, C.; Javitch, J.A.; Gether, U.; Egebjerg, J.; Christensen, K.V. Recruitment of β-arrestin2 to the dopamine D2 receptor: Insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 2008, 54, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Filtz, T.M.; Artymyshyn, R.P.; Guan, W.; Molinoff, P.B. Paradoxical regulation of dopamine receptors in transfected 293 cells. Mol. Pharmacol. 1993, 44, 371–379. [Google Scholar] [PubMed]
- Starr, S.; Kozell, L.B.; Neve, K.A. Drug-induced up-regulation of dopamine D2 receptors on cultured cells. J. Neurochem. 1995, 65, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Lachowicz, J.E.; Sibley, D.R. The D2S and D2L dopamine receptor isoforms are differentially regulated in Chinese hamster ovary cells. Mol. Pharmacol. 1994, 45, 878–889. [Google Scholar] [PubMed]
- Bischoff, S.; Krauss, J.; Grunenwald, C.; Gunst, F.; Heinrich, M.; Schaub, M.; Stocklin, K.; Vassout, A.; Waldmeier, P.; Maitre, L. Endogenous dopamine (DA) modulates [3H]spiperone binding in vivo in rat brain. J. Recept. Res. 1991, 11, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, C.; Bedard, P.J.; Falardeau, P.; Dipaolo, T. Behavioral and biochemical evidence for a different effect of repeated administration of l-DOPA and bromocriptine on denervated versus non-denervated striatal dopamine receptors. Neuropharmacology 1987, 26, 1601–1606. [Google Scholar] [CrossRef]
- Michelsen, K.; Yuan, H.; Schwappach, B. Hide and run. EMBO Rep. 2005, 6, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, M.; Haller, C.; Stoll, Y.; Abdel Aziz, S.; Biermann, B.; Mosbacher, J.; Kaupmann, K.; Bettler, B. The RXR-type endoplasmic reticulum-retention/retrieval signal of GABAB1 requires distant spacing from the membrane to function. Mol. Pharmacol. 2005, 68, 137–144. [Google Scholar] [PubMed]
- Shikano, S.; Li, M. Membrane receptor trafficking: Evidence of proximal and distal zones conferred by two independent endoplasmic reticulum localization signals. Proc. Natl. Acad. Sci. USA 2003, 100, 5783–5788. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, S.; Faron-Gorecka, A.; Dobrucki, J.; Polit, A.; Dziedzicka-Wasylewska, M. Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J. 2009, 276, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005, 26, 209–220. [Google Scholar] [CrossRef]
- O’Dowd, B.F.; Ji, X.; Nguyen, T.; George, S.R. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1–D2 heteromer formation. Biochem. Biophys. Res. Commun. 2012, 417, 23–28. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, B.F.; Nguyen, T.; Ji, X.; George, S.R. D5 dopamine receptor carboxyl tail involved in D5–D2 heteromer formation. Biochem. Biophys. Res. Commun. 2013, 431, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Hasbi, A.; O’Dowd, B.F.; George, S.R. Heteromeric dopamine receptor signaling complexes: Emerging neurobiology and disease relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Guiramand, J.; Montmayeur, J.P.; Ceraline, J.; Bhatia, M.; Borrelli, E. Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. J. Biol. Chem. 1995, 270, 7354–7358. [Google Scholar] [PubMed]
- Fan, Y.; Li, C.; Guo, J.; Hu, G.; Wu, G. A single Lys residue on the first intracellular loop modulates the endoplasmic reticulum export and cell-surface expression of α2A-adrenergic receptor. PLoS ONE 2012, 7, e50416. [Google Scholar] [CrossRef] [PubMed]
- Hurt, C.M.; Ho, V.K.; Angelotti, T. Systematic and quantitative analysis of G protein-coupled receptor trafficking motifs. Methods Enzymol. 2013, 521, 171–187. [Google Scholar] [PubMed]
- Sauvageau, E.; Rochdi, M.D.; Oueslati, M.; Hamdan, F.F.; Percherancier, Y.; Simpson, J.C.; Pepperkok, R.; Bouvier, M. CNIH4 interacts with newly synthesized GPCR and controls their export from the endoplasmic reticulum. Traffic 2014, 15, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Zerangue, N.; Malan, M.J.; Fried, S.R.; Dazin, P.F.; Jan, Y.N.; Jan, L.Y.; Schwappach, B. Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc. Natl. Acad. Sci. USA 2001, 98, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Macey, T.A.; Gurevich, V.V.; Neve, K.A. Preferential interaction between the dopamine D2 receptor and arrestin2 in neostriatal neurons. Mol. Pharmacol. 2004, 66, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Free, R.B.; Chun, L.S.; Moritz, A.E.; Miller, B.N.; Doyle, T.B.; Conroy, J.L.; Padron, A.; Meade, J.A.; Xiao, J.; Hu, X.; et al. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol. Pharmacol. 2014, 86, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Drinovec, L.; Kubale, V.; Nohr Larsen, J.; Vrecl, M. Mathematical models for quantitative assessment of bioluminescence resonance energy transfer: Application to seven transmembrane receptors oligomerization. Front. Endocrinol. 2012, 3, 104. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Liu, Y.; Bell, M.I.; Gurevich, V.V.; Neve, K.A. A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol. Pharmacol. 2009, 75, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Senogles, S.E. Investigation of the alternatively spliced insert region of the D2L dopamine receptor by epitope substitution. Neurosci. Lett. 2006, 393, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vickery, R.G.; von Zastrow, M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J. Cell Biol. 1999, 144, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aranda, M.F.; Acevedo, M.J.; Gutierrez, A.; Koulen, P.; Khan, Z.U. Role of a Gαi2 protein splice variant in the formation of an intracellular dopamine D2 receptor pool. J. Cell Sci. 2007, 120, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.M.; Liu, Y.; Vidensky, S.; Maier, S.; Jung, E.; Farhan, H.; Robinson, M.B.; Sitte, H.H.; Rothstein, J.D. The endoplasmic reticulum exit of glutamate transporter is regulated by the inducible mammalian Yip6b/GTRAP3–18 protein. J. Biol. Chem. 2008, 283, 6175–6183. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, N.; Woll, M.P.; Nordman, J.C.; Levenson, R. Dopamine receptor interacting proteins: Targeting neuronal calcium sensor-1/D2 dopamine receptor interaction for antipsychotic drug development. Curr. Drug Targets 2012, 13, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, E.; Fontaine, V.; Picetti, R.; Lombardi, M.; Samad, T.A.; Oulad-Abdelghani, M.; Edwards, R.; Borrelli, E. Signaling by dopamine regulates D2 receptors trafficking at the membrane. Cell Cycle 2008, 7, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yamamoto, Y.; Watanabe, M.; Binas, B.; Owada, Y.; Fukunaga, K. Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J. Neurosci. 2010, 30, 3146–3155. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Vrecl, M.; Anderson, L.; Hanyaloglu, A.; McGregor, A.M.; Groarke, A.D.; Milligan, G.; Taylor, P.L.; Eidne, K.A. Agonist-induced endocytosis and recycling of the gonadotropin releasing hormone receptor: Effect of β-arrestin on internalization kinetics. Mol. Endocrinol. 1998, 12, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Drinovec, L.; Glisic, S.; Veljkovic, N.; Nohr, J.; Vrecl, M. Demonstration of a direct interaction between β2-adrenergic receptor and insulin receptor by BRET and bioinformatics. PLoS ONE 2014, 9, e112664. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2016. Available online: http://imagej.nih.gov/ij/ (accessed on 30 June 2016).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.; Kellett, E.; McVey, M.; Rees, S.; Milligan, G. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): Hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem. J. 2002, 365, 429–440. [Google Scholar] [PubMed]




| Construct | IC50 (nM) | Bmax (Fold Change) |
|---|---|---|
| D2L-R | 0.82 ± 0.17 | 1.00 ± 0.00 |
| M1 | 1.84 ± 0.03 | 2.20 ± 0.54 |
| M2 | 1.11 ± 0.19 | 1.84 ± 0.33 |
| M3 | 1.74 ± 0.29 | 2.27 ± 0.36 |
| M4 | 1.26 ± 0.03 | 2.15 ± 0.77 |
| M5 | 1.83 ± 0.60 | 2.94 ± 0.48 |
| M6 | 2.40 ± 0.02 | 3.72 ± 1.40 |
| M7 | 2.93 ± 1.41 | 3.40 ± 2.03 |
| Construct | BRETmax (Fold Change) | EC50 (nM) | IC50 (nM) |
|---|---|---|---|
| D2L-R/RLuc8 | 1.00 ± 0.10 | 140.5 ± 31.6 | 149.7 ± 54.4 |
| M1/RLuc8 | 1.41 ± 0.03 * | 232.1 ± 73.1 | 99.5 ± 10.1 |
| M2/RLuc8 | 1.51 ± 0.10 * | 222.8 ± 62.2 | 129.9 ± 16.8 |
| M3/RLuc8 | 1.44 ± 0.07 * | 183.6 ± 28.1 | 107.1 ± 24.1 |
| M4/RLuc8 | 1.54 ± 0.09 * | 218.3 ± 40.7 | 98.8 ± 9.1 |
| M5/RLuc8 | 1.51 ± 0.07 * | 208.8 ± 45.4 | 100.7 ± 37.5 |
| M6/RLuc8 | 1.41 ± 0.09 * | 318.8 ± 145.2 | 96.1 ± 30.5 |
| M7/RLuc8 | 1.31 ± 0.05 | 327.9 ± 119.4 | 75.8 ± 23.9 |
| Construct | EC50(nM) | Inhibition of Forskolin—Induced cAMP Accumulation (Fold Change) |
|---|---|---|
| D2L-R/RLuc8 | 1.88 ± 0.57 | 1.00 ± 0.04 |
| M1/RLuc8 | 3.68 ± 0.55 | 2.09 ± 0.21 |
| M2/RLuc8 | 2.45 ± 0.87 | 1.74 ± 0.22 |
| M3/RLuc8 | 2.82 ± 0.66 | 2.96 ± 0.35 * |
| M4/RLuc8 | 2.17 ± 0.96 | 2.91 ± 0.48 * |
| M5/RLuc8 | 1.15 ± 0.68 | 4.46 ± 0.81 * |
| M6/RLuc8 | 2.82 ± 0.89 | 2.85 ± 0.59 * |
| M7/RLuc8 | 1.91 ± 1.2 | 1.68 ± 0.22 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubale, V.; Blagotinšek, K.; Nøhr, J.; Eidne, K.A.; Vrecl, M. The Conserved Arginine Cluster in the Insert of the Third Cytoplasmic Loop of the Long Form of the D2 Dopamine Receptor (D2L-R) Acts as an Intracellular Retention Signal. Int. J. Mol. Sci. 2016, 17, 1152. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071152
Kubale V, Blagotinšek K, Nøhr J, Eidne KA, Vrecl M. The Conserved Arginine Cluster in the Insert of the Third Cytoplasmic Loop of the Long Form of the D2 Dopamine Receptor (D2L-R) Acts as an Intracellular Retention Signal. International Journal of Molecular Sciences. 2016; 17(7):1152. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071152
Chicago/Turabian StyleKubale, Valentina, Kaja Blagotinšek, Jane Nøhr, Karin A. Eidne, and Milka Vrecl. 2016. "The Conserved Arginine Cluster in the Insert of the Third Cytoplasmic Loop of the Long Form of the D2 Dopamine Receptor (D2L-R) Acts as an Intracellular Retention Signal" International Journal of Molecular Sciences 17, no. 7: 1152. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071152






